Servicios Personalizados
Revista
Articulo
Indicadores
Compartir
Journal of Human Growth and Development
versión impresa ISSN 0104-1282versión On-line ISSN 2175-3598
J. Hum. Growth Dev. vol.31 no.3 Santo André sep./dic. 2021
https://doi.org/10.36311/jhgd.v31.12782
ORIGINAL ARTICLE
DOI: 10.36311/jhgd.v31.12782
COVID-19 and its relationship with kidney diseases: a scope review
Jennifer Soanno MarchioriI; Miguel Athos Da Silva De OliveiraI; Italla Maria Pinheiro BezerraII
IStudents of the 10th period of the Undergraduate Nursing Course, Escola Superior de Ciências da Santa Casa de Misericórdia de Vitória EMESCAM - Vitória, Espírito Santo, Brazil
IINurse. PhD in Health Sciences from the Centro Universitário FMABC. Professor of the Undergraduate Nursing Course at Escola Superior de Ciências da Santa Casa de Misericórdia de Vitória EMESCAM - Vitória, Espírito Santo, Brazil
ABSTRACT
BACKGROUNG: COVID-19 is an acute respiratory disease originally from China that emerged in December 2019 and quickly spread around the world, affecting 230,418.415 people, and causing 4,724,876 deaths. Coming from the coronavirus family, SARS-CoV-2 is a new subtype of virus that affects the respiratory tract in different levels and can spread and affect other vital structures in the body.
OBJECTIVE: to identify the risk factors that lead patients infected by the new coronavirus to develop kidney disease.
METHODS: this is a systematic review of the Scoping Review type (scope review), according to the method proposed by the Joanna Briggs Institute, with the implementation of a checklist structured by PRISMA-ScR that contains 22 mandatory items. The following descriptors were used: coronavirus infection, acute kidney injury and risk factors in five databases, namely PudMed, Scopus, Embase, Virtual Health Library and Web of Science.
RESULTS: while reading the studies, it was concluded that Acute Kidney Injury was the main renal finding in patients contaminated by SARS-CoV-2. The risk factors for developing renal worsening in patients with COVID-19 were the extremes of age, race, sex, pre-existing diseases, and the disease evolution.
CONCLUSION: it is assumed that renal involvement does not occur only for an exclusive reason, but as a set of factors. It is up to the health team to pay constant attention to the warning signs by monitoring the contaminated patient.
Keywords: Coronavirus infection, acute kidney injury, risk factors.
Authors summary
Why was this study done?
This study conducted was made thinking about the level of incidence and registraition of kidney comitment in patient with COVID-19 and what was the cause as well, considerating that this disease affects majoritaryly the respiratory tract.
What did the researchers do and find?
The aims of this study is identify the risk factors that lead patients infected by the new coronavirus to develop kidney disease. A review was carried out based on the literature available in the databases. The majoritary findings concluded that Acute Kidney Injury (AKI) was the main renal finding in patients contaminated by SARS-coV-2, follow by the risk factors identified for developing renal worsening in patients with COVID-19, like the extremes of age, race, sex, pre-existing diseases and disease evolution.
What do these findings mean?
The renal involvement does not occur only for an exclusive reason, but as a set of factors surrounding the corse of the desease and the biological profil of the patient.
INTRODUCTION
COVID-19 is an acute respiratory disease caused by SARS-CoV-2 that has spread rapidly around the world, originating in Hubei Province, Wuhan, China, in December 2019. This new disease is not the first of its family, but a variation of the coronavirus family that rarely affects humans, being confined only to animals such as bats, cattle, cats, and camels, such as MERS-CoV and SARS-CoV. Although the exact origin of SARS-CoV-2 is not known, it is understood that its viral structure is 96.2% similar to CoV-RaTG13, which mainly affects bats and is 79.5% like SARS-CoV than the other presented forms of the coronavirus family1-3.
In January 2020, the World Health Organization (WHO) declared the outbreak of COVID-19 as the sixth public health emergency of international attention, in which health workers, governments and the general population in order that the spread of the disease was prevented. As of February 2020, WHO reported 45,171 cases and 1,114 deaths, of which 99% of cases and 99% of deaths were related to COVID-19 in China. The spread of the disease occurred rapidly and exponentially, reaching almost all countries and, even with the attempt to contain the disease, the WHO declared in March 2020, a pandemic caused by SARS-CoV-22-4.
SARS-CoV-2 is the virus that causes the COVID-19 disease, which mainly affects the respiratory tract initially with a flu-like condition, with the most common symptoms such as dry cough, runny nose, fever, headache, myalgia, sore throat and, later, anosmia (loss of smell), ageusia (taste alteration), which lead to hyporexia (decreased appetite), with the possibility of progressing to severe pneumonia, with difficulty breathing, dyspnea (shortness of breath), and asthenia (tiredness). Among the symptoms presented by people infected by SARS-CoV-2, many may present gastrointestinal disorders (diarrhea, nausea/vomiting), even though they are not the most common. The transmission of the new virus occurs through the respiratory tract and/or mucous membranes, with dispersion by droplets, respiratory secretions, and direct contact3,5.
COVID-19 can present itself in 80% of cases in an asymptomatic or oligosymptomatic form (mild symptoms) and, in 20% of cases, it evolves to the severe form of the disease, requiring respiratory support in 5% of cases. Acute respiratory failure syndrome is a complication resulting from COVID-19, which occurs more often in the elderly, immunosuppressed people, or those with other comorbidities, such as hypertension, diabetes, neurological and respiratory diseases. These risk factors, or their accumulation, are the main findings in the emergence of complications and mortality rate6.
SARS-CoV-2 is found in the respiratory tract secretions, saliva, feces and urine in patients with diarrhea. The onset of symptoms occurs from the 3rd to the 14th day after infection. Its diagnosis is given through laboratory, clinical-imaging, clinical-epidemiological and clinical tests, however, there is no treatment, only vaccines control the disease4-6.
Renal function is a fundamental part of the functioning of the human body; any injury can harm this cycle and disrupt human metabolism. With acute kidney injury (AKI) as a multifactorial impairment of rapid evolution in renal filtration function, the factors that lead to the development of AKI revolve around preexisting clinical diseases, susceptibility and some therapeutic interventions, however its appearance may vary between stages , such as pre-renal, renal and post-renal, caused by decreased renal perfusion or hypotensive drugs, direct damage to glomerular, tubular or tubulointestinal structures, and obstruction of the flow of urine in renal structures to the urethra, respectively7-9.
Thus, the study problem is: What are the risk factors that lead the relationship of patients with COVID-19 to develop kidney disease? Thus, the objective of the study is to identify the risk factors that lead the patient contaminated by SARS-CoV-2 to develop kidney diseases.
It is believed that this study becomes relevant, as it makes known the current studies on the subject, defining the reason why so many patients with COVID-19 evolve to some level of renal involvement and enabling the guidance of professionals on the necessary management.
METHODS
This is a systematic review of the Scoping Review type in accordance with the review method proposed by the Joanna Briggs Institute (JBI). The scope review is intended to map, through a transparent and rigorous method, the main concepts of a given area of knowledge, bringing a complete view, to compile and disseminate the data obtained and identify gaps in existing research without evaluating them critically10.
The research question was made using the PCC acronym (Population, Concept, Context): Population - people affected by COVID-19; Concept - kidney disorders; Context - relationship of COVID-19 with kidney diseases.
The study was based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Review (PRISMA-ScR) Checklist, a guiding script to carry out a scope review built through the guidelines of the Joanna Briggs Institute (JBI). PRISMA-ScR presents 22 separate items according to the mandatory chapters in the review, such as: title, abstract, introduction, method, results, discussions and funding11.
Thus, the search for relevant studies was carried out through the PudMed, Scopus, Embase, VHL (Virtual Health Library) and Web of Science databases. The bibliographic survey took place from April to July 2021, using the double-checking method.
Descriptors were identified according to the research theme, delimited through MeSh (Medical Subject Headings) and DeCS (Descriptors in Health Sciences). The search strategies were used in English and Portuguese "coronavirus infection AND acute kidney injury AND risk factors".
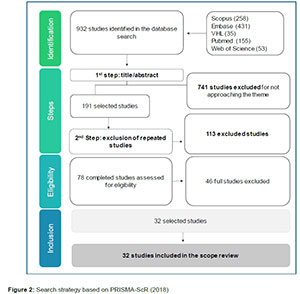
To carry out the search in the databases, studies in English and Portuguese were included, in which review studies, case studies, experience reports, editorials, letters, theses, dissertations and course conclusion studies were excluded. The research followed the flow exemplified below (figure 1):
RESULTS
A total of 32 studies were selected, which underwent the process of reading titles, abstracts, application of eligibility criteria and full reading. Initially, 932 studies were identified and 690 of these were excluded by title reading, 51 by abstract reading and 46 by full reading, as shown in the figure below.
According to the survey in the databases, 32 articles that addressed risk factors related to kidney disease in patients with COVID-19 were part of this review. The first 20 articles were published in 2020, showing the impact of this new disease and the efforts of the scientific community to understand it.
Most articles addressed the disease evolution process in patients affected by COVID-19, as well as the management and identification of the virulent condition, in addition to highlighting possible causes that would lead to the development of Acute Kidney Injury (AKI). The articles also address risk factors for kidney involvement, bringing a discussion focused on possible biomarkers of some degree of kidney injury, such as: laboratory test results, immunological markers, pre-existing diseases, and the individual's biological characteristics.
Thus, for a better organization of this review, it was decided to separate the main findings by topics, following the logic of the individual's trajectory in the disease.
Laboratory findings signaling of kidney injury
Table 1 shows the results that addressed the laboratory analyses. Most authors agreed that the findings represent markers for renal involvement during COVID-19, namely: systemic inflammation markers (serum procalcitonin and blood leukocytes); Estimated Gromerular Filtration Rate (GFR); low serum albumin; lymphopenia; high D-dimer; C-reactive protein (CRP); proteinuria; Soluble urokinase-type plasminogen activator receptor (suPAR); high platelet count; hyperinflammation; tendency to coagulopathy; high lactose dehydrogenase levels; and elevated serum creatinine.
Immunological manifestations related to SARS-COV-2 contamination
Table 2 shows the results that addressed immunological markers in patients affected by COVID-19. The main findings are: Elevated levels of IL8, IL10 and IL2R.
Pre-existing diseases as a bad prognosis in kidney injury during covid-19
The results that mostly addressed pre-existing diseases of patients who were affected by COVID-19 are shown in table 3. During the data analysis, it was evident that the pre-existing conditions that most cause the development of some type of injury kidney disease in the onset of that disease are: hypertension; diabetes; cardiac insufficiency; peripheral vascular disease; other cardiovascular diseases; Chronic Kidney Disease (CKD); other pre-existing kidney diseases; high body mass index (BMI); hematologic malignancy; immunosuppression in general; and cerebrovascular diseases.
Biological aspects facing kidney injury at covid-19
Table 4 presents results of biological factors that cause the development of some stage of Acute Kidney Injury (AKI) during the onset of COVID-19. They are elderly, male and African descent.
Trajectory of the patient with covid-19, its evolution and renal impact
According to table 5, the results address the evolution of the disease as a causative factor for kidney injury. It is a fact that AKI is the most recurrent involvement in patients affected by COVID-19. This fact is associated with the use of invasive mechanical ventilation (IMV); vasopressor drugs; nephrotoxic drug; high APACHE II score; diuretics; heart failure; drop in PaO2/FiO2; sepsis; and renal replacement therapy (RRT) of some kind, such as dialysis. This direct relationship with the evolution to LRA is indicated.
Another data identified corresponds to the period of hospitalization and diagnosis of AKI in patients positive for COVID-19. Most individuals admitted to the ICU with some degree of renal impairment do not survive after a period of 28 days, in part due to the virulent power of SARS-CoV-2, or due to missed diagnoses. As for the raised histopathological finding, AKI may occur due to acute tubular injury, possibly caused by the direct virulence of SARS-CoV-2 in the proximal tubular epithelium, since virus particles were observed in the tubular epithelium and podocytes.
DISCUSSION
COVID-19 is considered a respiratory disease caused by the SARS-CoV-2 virus, which leads the individual to develop an acute respiratory syndrome. With its spiral shape and the presence of Spike (S) protein around it, SARS-CoV-2 attacks alveolar epithelial cells through the angiotensin-2 converting enzyme (ACE-2) through the protein43,44.
Although COVID-19 mainly affects the lungs, other organs are affected in the course of the disease, especially the heart, liver, intestine, brain, testicles, and kidneys. The reason why other organs are equally attacked is because of the presence of ACE-2 in cells, such as the continuous circulation of this enzyme in the bloodstream43.
In the case of kidney disease, according to the data obtained, its identification as Acute Kidney Injury (AKI) was unanimous in the studies analyzed, considering that the kidneys are one of the main organs affected by SARS-CoV-2. For a better understanding of this comorbidity, it is important to mention that the diagnosis of AKI is given by the criteria described in the manual of the organization Kidney Disease Improving Global Outcomes (KDIGO)45.
AKI is an acute kidney disease that is divided into 3 stages, identified through the quantification of serum creatinine and excreted urine. The stage 1 patient has 1.5-1.9 times the baseline or ≥0.3mg/dl (≥26.5mmol/l) increase in serum creatinine, and urine quantitation <0.5ml/kg/h between 6-12 hours; stage 2, which presents 2.0-2.9 times the baseline serum creatinine and <0.5ml/kg/h for ≥12 hours of quantified urine; stage 3, for those who present serum creatinine in 3.0 times the baseline or increase in serum creatinine to ≥4.0mg/dl (≥353.6mmol/l) and urine quantification <0.3ml/kg/h for ≥24 hours or anuria for 12 hours46.
It is noteworthy that patients starting renal replacement therapy or those under 18 years old who show a decrease in GFR to <35ml/min per 1.73m2 also fall into stage 3 of AKI46.
Regarding the importance of laboratory findings for diagnosing acute kidney injury, tests performed for the analysis of some biological materials, such as blood and urine, in order to make a diagnosis or just monitor the functioning of the body47. Considering that physiological changes often alter laboratory patterns, with patients affected by COVID-19, it is no different.
According to the results obtained, it is possible to affirm that patients contaminated by SARS-CoV-2 have some laboratory alterations that can serve as indicators of an AKI, namely: serum albumin <3.5g/dl, lymphopenia, thrombocytosis, hyperferritinemia , serum creatinine >1.3mg/dl, proteinuria, gromerular filtration rate (GFR) <60ml/min/1.73m2, elevated C-reactive protein (CRP) level, D-dimer >0.500µd/ml and lactate dehydrogenase 246 IU/L15-17,19,20,40.
Still thinking about the laboratory alterations presented by patients with COVID-19, it is known that the soluble urokinase-type plasmogen activator receptor (suPAR) and serum procalcitonin, among others, are also biomarkers associated with the onset of AKI18,13.
Among the laboratory changes to be observed, it is important to highlight the role of thrombocytosis, fibrinogen and D-dimer in the prognosis of patients affected by COVID-19, as this finding is important when identifying several cases of thromboembolism, which comes as a possible factor for the LRA20,48. Still, other studies address the disagreement with the role of ferritin, CRP, D-dimer and fibrinogen in the role of identifiable measurable markers for the detection of AKI12.
Such findings contribute to the primary signaling of physiological changes in the patient's body, requiring the attention of active health professionals.
Although COVID-19 is a new disease, the way the body behaves is no different. With the activation of the immune system at the first sign of an "invader". The identification of interleukins at an increased level in patients who presented AKI is responsible for the pro-inflammatory action against SARS-CoV-2, presented by IL-6, IL-8, IL-2R, and later IL-10, IL- 1β19,21. Such identification leads one to believe that these proteins command the immune system and cause a storm of cytokines, which reflects in an exacerbated action of the individual's immune system, causing a protective factor to become harmful, thus interfering with good patient recovery. Cytokine storms, together with other factors such as elevated D-dimer, are related to the formation of blood thrombi, which act as a potential cause of AKI against the complication and evolution of COVID-19 in infected patients49,50.
Other studies claim that immunobiological identification does not always come as a biological marker signaling the decline in renal function, but only as a clinical finding of the human body's reaction to viral infection12. These high levels of protein concentration are important indicators in the follow-up of patients in severe cases of the disease.
It is understood that some chronic diseases already act as a risk factor in infectious diseases in general. In the COVID-19 pandemic, this fact was increasingly discussed, making it clear that care for these people should be redoubled5. This relationship happens because patients with chronic diseases have a greater amount of the ACE-2 enzyme expressed in their body. Thus, due to the great affinity of SARS-CoV-2 with this enzyme, the high risk that carriers have is understandable51.
Based on the findings during the searches, some pre-existing diseases are a risk during the evolution of COVID-19, such as cardiovascular diseases (heart failure and peripheral vascular disease), hypertension, diabetes mellitus, immunosuppression by any factor, chronic kidney disease, cerebrovascular diseases, and high body mass index (BMI)22-27. There are also other conditions that qualify as a risk factor, but which were not identified in the studies evaluated, which are: smoking, pregnancy, asthma, chromosomal diseases in a state of immunological weakness, chronic obstructive pulmonary disease (COPD) and hematological diseases (anemia)5.
The relationship between pre-existing diseases and COVID-19 is due to the way in which the body responds to contamination by SARS-CoV-2, considering that an individual who has a chronic disease, or factors that compromise full functioning immune system, are more vulnerable to a poor prognosis during contamination and recovery. The fact to be raised is not only about the risk that this individual is predisposed to, but about how the body will respond to the new virus and, consequently, how the disease evolves. The more vulnerable the patient, the more likely he is to develop a more severe condition in COVID-19 and the greater the risk of developing AKI25,52.
Therefore, it is important to maintain close surveillance for this type of patient, as they are more likely to develop kidney damage from COVID-19.
It is known that biological aspects can influence the onset of AKI in patients infected with SARS-CoV-2. Age, gender and ethnic characteristics may be the characteristics of those who demonstrate a great impact in the search for factors that permeate AKI patients in COVID-19.
According to the data obtained, individuals over 60 years of age and male are the most affected. Being elderly has always been a vulnerability factor, as the body no longer has the same appearance as youth, the physiological course no longer works as it used to and chronic diseases arrive, and by itself the body loses a little more of its defense53,54. COVID-19 presents itself more aggressively in older people, its relationship may be linked to a decline in the immune system, as younger patients infected by the virus have a better prognosis and a lower rate of AKI development55.
The relationship between the prevalence of males in AKI cases in patients contaminated by SARS-CoV-2 is not clear, although studies show that males take care less of their own health, arriving at the health service most of the time with a more advanced disease picture56.
When related to the fact that people of African descent are more likely to have an increased risk for AKI during COVID-19. This is because the individual is structurally more likely to have hematological diseases, hypertension and diabetes28,31,56,57. Also regarding ethnic characteristics, within the samples of selected studies, there was a discrepancy in the percentage of people who developed AKI during COVID-19. Those coming from the West had almost three times more individuals with some degree of AKI than those from the East. This may be related to the fact that there is a greater expression of the ACE-2 enzyme in the podocytes and proximal tubule of individuals from the West58.
In general, all factors lead to some pre-existing disease in the infected patient, thus explaining why people with such characteristics are more likely to develop AKI during COVID-19.
AKI does not always start in a hospital setting. There are some acute kidney injuries of pre-renal origin, that is, that developed in a community, either by dehydration or other factors, but which became more significant when combined with COVID-19. Through the study, the equivalence between pre-renal and intrinsic AKI (which originated in a hospital environment) was evident, with an equal risk of mortality for both32.
Of all stages of AKI, stage 3 is the most dangerous, as it is when there is no possibility of renal capacity returning, unlike stages 1 and 2. Patients who have AKI 3 concomitantly with COVID-19 do not showed improvement in renal function and/or did not survive a period of more than 28 days of hospitalization, with variable outcomes between Continuous Renal Replacement Therapy (TSRC), organ transplantation and death31,34.
The use of early RRT in patients with developing AKI can bring benefits, as substances harmful to the body will be removed and the amount of excess fluid in that body will decrease35. On the other hand, there is a significant increase in the risk of death in patients who underwent dialysis36.
There are some factors that increase the patient's chance of developing AKI, such as: drop in PaO2/FiO2, cardiopulmonary arrest, secondary infections, as well as the necessary approaches during the care of patients affected by COVID-19 that become risk factors for the emergence of AKI, such as: Invasive Mechanical Ventilation (IMV), admission and stay in the ICU, use of diuretics, vasopressor drugs and other nephrotoxic drugs31,37,41,39. In contrast, although IMV is a potential risk factor for the development of AKI and, consequently, death, the "delay" in performing the intubation would be the most harmful for these patients59.
As kidneys have a large amount of the ACE-2 enzyme in their tissue, the histopathological findings of the presence of the virus itself in the proximal tubule are a possible migration of SARS-CoV-2 through the bloodstream, facilitated by the circulating ACE-224,42.
It is important that AKI is identified early, in order to intervene and interrupt the progressive worsening trajectory of renal involvement. It is worth considering a worrying factor that provides an increase in the mortality rate: the lack of diagnoses in patients admitted to the ICU who already show signs of acute renal failure. The body demonstrates the signs of AKI and it is up to the professional to investigate and draw a line of care in order to increase the life span of this patient, as well as to preserve as much as possible the renal function of that individual hospitalized for COVID-1927.
The occurrence of Acute Kidney Injury (AKI) in patients contaminated by SARS-CoV-2 is still not clear, however, it is believed that this pathology will present itself as a sentinel factor signaling a sequential failure of organs, or the consequence itself of a multiple organ failure40.
Through the analysis of the articles, it was possible to understand that the critical condition of patients with COVID-19 is one of the main factors for renal impairment.
Although some studies address the formation of blood thrombi as one of the main causes of AKI in patients affected by COVID-19, the direct action of the virus on the renal epithelia must be considered. Thus, the need for further studies is clear. It is important to consider the hypothesis that renal impairment does not come from an exclusive reason, but as a combination of factors, such as the drug approach, physiological changes and other factors that harm the renal system.
The healthcare team must be constantly on the lookout for all warning signs for a patient in serious condition due to COVID-19. Early diagnosis can abruptly change the course of the disease and allow the best possible intervention for the individual.
CONCLUSION
It was evidenced that the risk factors that lead the patient contaminated by SARS-CoV-2 to develop kidney diseases were age, male gender, hypertension, diabetes, heart failure, immunosuppression, cerebrovascular diseases, chronic kidney disease, obesity, African descent, use of mechanical ventilation, sepsis and use of diuretics.
Regarding the relationship of patients with COVID-19 and the development of kidney diseases, the age and gender factor of individuals affected by the disease indicate a higher incidence of AKI. This is followed by the observance of pre-existing diseases, such as hypertension and diabetes, which call for attention in monitoring the evolution of the disease. The use of mechanical ventilation and sepsis are also highlighted as preponderant factors in the development of AKI and/or death.
Author Contributions
JSM, Project and manuscript elaboration; MASO, data collection and organization; IMPB, final review of material.
Funding
no financial support
Acknowledgments
to the Escola Superior de Ciências da Santa Casa de Misericórdia de Vitória - EMESCAM.
Conflicts of Interest
no conflicts of interest.
REFERENCES
1.Ministério da Saúde (BR). 1. ed. Guia de Vigilância Epidemiológica: Emergência de Saúde Pública de Importância Nacional pela Doença pelo Coronavírus 2019. 2020. [ Links ]
2.Kannan S, Shaik Syed Ali P, Sheeza A, Hemalatha K. COVID-19 (Novel Coronavirus 2019) - recent trends. European Review for Medical and Pharmacological Sciences. fevereiro de 2020; 24(4): 2006-11. DOI: 10.26355/eurrev_202002_20378. 1 [ Links ]
3.Guo Y-R, Cao Q-D, Hong Z-S, Tan Y-Y, Chen S-D, Jin H-J, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status. Military Med Res. Dezembro de 2020; 7(1): 11. DOI: 10.1186/s40779-020-00240-01. [ Links ]
4.Freitas AS, Zica GM, Albuquerque CL de. Pandemia de coronavírus (COVID-19): o que os fonoaudiólogos devem saber. CoDAS. 2020; 32(3): e20200073. [ Links ]
5.Ministério da Saúde (BR). Sobre a Doença. 2020. Avaliable from: https://coronavirus.saude.gov.br/ [ Links ]
6.World Health Organization (WHO). COVID-19 Vaccines. 2021. Avaliable from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19-vaccines [ Links ]
7.Benichel CR, Meneguin S. Fatores de risco para lesão renal aguda em pacientes clínicos intensivos. Acta Paulista de Enfermagem. 11 de março de 2020; 33: e-APE20190064. [ Links ]
8.Gabarre P, Dumas G, Dupont T, Darmon M, Azoulay E, Zafrani L. Acute kidney injury in critically ill patients with COVID-19. Intensive Care Med. julho de 2020; 46(7): 1339-48. DOI: 10.1007/s00134-020-06153-9 [ Links ]
9.Ribeiro, GLH, da Rosa, AF, Florian, PZ, Antonello, ICF. Lesão renal aguda. Acta méd. Porto Alegre. 2016, 6-6. [ Links ]
10.Peters M, Godfrey C, McInerney P, Munn Z, Trico A, Khalil H. Chapter 11: Scoping Reviews. In: Aromataris E, Munn Z, organizadores. JBI Manual for Evidence Synthesis [Internet]. JBI; 2020 [citado 12 de Julho de 2020]. Avaliable from: https://wiki.jbi.global/display/MANUAL/Chapter+11%3A+Scoping+reviews [ Links ]
11.Tricco, AC, Lillie, E, Zarin, W, O'Brien, KK, Colquhoun, H, Levac, D, ... & Straus, SE. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Annals of internal medicine, 2018; 169(7), 467-473. [ Links ]
12.Ghosn M, Attallah N, Badr M, Abdallah K, De Oliveira B, Nadeem A, et al. Severe Acute Kidney Injury in Critically Ill Patients with COVID-19 Admitted to ICU: Incidence, Risk Factors, and Outcomes. JCM. 15 de março de 2021 ;10(6): 1217. [ Links ]
13.Hardenberg J-HB, Stockmann H, Aigner A, Gotthardt I, Enghard P, Hinze C, et al. Critical Illness and Systemic Inflammation Are Key Risk Factors of Severe Acute Kidney Injury in Patients With COVID-19. Kidney International Reports. Abril de 2021; 6(4): 905-15. [ Links ]
14.Wang J, Wang Z, Zhu Y, Li H, Yuan X, Wang X, et al. Identify the Risk Factors of COVID-19-Related Acute Kidney Injury: A Single-Center, Retrospective Cohort Study. Front Med. 28 de julho de 2020; 7: 436. [ Links ]
15.Lim J-H, Park S-H, Jeon Y, Cho J-H, Jung H-Y, Choi J-Y, et al. Fatal Outcomes of COVID-19 in Patients with Severe Acute Kidney Injury. JCM. 3 de junho de 2020; 9(6) :1718. [ Links ]
16.Neves PD, Sato V, Mohrbacher S, Ferreira B, Pereira LV, Oliveira ES, Chocair P, et al. AKI due to COVID-19 in the intensive care unit: Analysis of a Brazilian Center. Journal of the American Society of Nephrology, 2020; 252-252. [ Links ]
17.Ouahmi H, Courjon J, Morand L, François J, Bruckert V, Lombardi R, et al. Proteinuria as a Biomarker for COVID-19 Severity. Front Physiol. 9 de março de 2021; 12: 611772. [ Links ]
18.Azam TU, Shadid HR, Blakely P, O'Hayer P, Berlin H, Pan M, et al. Soluble Urokinase Receptor (SuPAR) in COVID-19-Related AKI. JASN. Novembro de 2020; 31(11): 2725-35. [ Links ]
19.Xia P, Wen Y, Duan Y, Su H, Cao W, Xiao M, et al. Clinicopathological Features and Outcomes of Acute Kidney Injury in Critically Ill COVID-19 with Prolonged Disease Course: A Retrospective Cohort. Journal of the American Society of Nephrology. Setembro de 2020; 31(9): 2205-21. [ Links ]
20.Cheng Y, Luo R, Wang X, Wang K, Zhang N, Zhang M, et al. The Incidence, Risk Factors, and Prognosis of Acute Kidney Injury in Adult Patients with Coronavirus Disease 2019. CJASN. 7 de outubro de 2020; 15(10): 1394-402. [ Links ]
21.Cheng Y, Zhang N, Luo R, Zhang M, Wang Z, Dong L, et al. Risk Factors and Outcomes of Acute Kidney Injury in Critically Ill Patients with Coronavirus Disease 2019. Kidney Dis. 26 de outubro de 2020; 7(2): 111-119. [ Links ]
22.Phillips T, Stammers M, Leggatt G, Bonfield B, Fraser S, Armstrong K, et al. Acute kidney injury in COVID-19: Identification of risk factors and potential biomarkers of disease in a large UK cohort. Nephrology. Maio de 2021; 26(5): 420-31. [ Links ]
23.Gasparini M, Khan S, Patel JM, Parekh D, Bangash MN, Stϋmpfle R, et al. Renal impairment and its impact on clinical outcomes in patients who are critically ill with COVID-19: a multicentre observational study. Anaesthesia. Março de 2021; 76(3): 320-6. [ Links ]
24.Xiao G, Hu H, Wu F, Sha T, Zeng Z, Huang Q, Li H, Han J, Song W, Chen Z, Cai S. Acute kidney injury in patients hospitalized with COVID-19 in Wuhan, China: a single-center retrospective observational study. Journal of Southern Medical University. Fevereiro de 2021; 41(2): 157-163. DOI: 10.12122/j.issn.1673-4254.2021.02.01 [ Links ]
25.Phillips T, Leggatt G, Stammers M, Armstrong K, Fraser SD, Bonfield B, Veighey K. COVID-19 AKI : Risk factors and markers of disease from a large UK cohort. Journal of the American Society of Nephrology. 2020; 31: 250 [ Links ]
26.Lowe R, Ferrari M, Nasim-Mohi M, Jackson A, Beecham R, Veighey K, et al. Clinical characteristics and outcome of critically ill COVID-19 patients with acute kidney injury: a single centre cohort study. BMC Nephrol. Dezembro de 2021; 22(1): 92. [ Links ]
27.Li Q, Zhang T, Li F, Mao Z, Kang H, Tao L, et al. Acute Kidney Injury Can Predict In-Hospital Mortality in Elderly Patients with COVID-19 in the ICU: A Single-Center Study. CIA. Novembro de 2020; 15: 2095-107. [ Links ]
28.Nimkar A, Naaraayan A, Hasan A, Pant S, Durdevic M, Suarez CN, et al. Incidence and Risk Factors for Acute Kidney Injury and Its Effect on Mortality in Patients Hospitalized From COVID-19. Mayo Clinic Proceedings: Innovations, Quality & Outcomes. Dezembro de 2020; 4(6): 687-95. [ Links ]
29.Dai Y, Liu Z, Du X, Wei H, Wu Y, Li H, et al. Acute Kidney Injury in Hospitalized Patients Infected with COVID-19 from Wuhan, China: A Retrospective Study. Iannotti F, organizador. BioMed Research International. 11 de janeiro de 2021; 2021: 1-8. [ Links ]
30.Zahid U, Ramachandran P, Spitalewitz S, Alasadi L, Chakraborti A, Azhar M, et al. Acute Kidney Injury in COVID-19 Patients: An Inner City Hospital Experience and Policy Implications. Am J Nephrol. 2020; 51(10): 786-96. [ Links ]
31.31 Hirsch JS, Ng JH, Ross DW, Sharma P, Shah HH, Barnett RL, et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney International. Julho de 2020; 98(1): 209-18. [ Links ]
32.Hansrivijit P, Gadhiya KP, Gangireddy M, Goldman JD. Risk Factors, Clinical Characteristics, and Prognosis of Acute Kidney Injury in Hospitalized COVID-19 Patients: A Retrospective Cohort Study. Medicines. 7 de janeiro de 2021; 8(1): 4. [ Links ]
33.Diebold M, Schaub S, Landmann E, Steiger J, Dickenmann M. Acute kidney injury in patients with COVID-19: a retrospective cohort study from Switzerland. Swiss Med Wkly. 1º de março de 2021. Avaliable from: https://doi.emh.ch/smw.2021.20482 [ Links ]
34.Xu J, Xie J, Du B, Tong Z, Qiu H, Bagshaw SM. Clinical Characteristics and Outcomes of Patients With Severe COVID-19 Induced Acute Kidney Injury. J Intensive Care Med. Março de 2021; 36(3): 319-26. [ Links ]
35.Zamoner W, Santos CA da S, Magalhães LE, Oliveira PGS de, Balbi AL, Ponce D. Acute Kidney Injury in COVID-19: 90 Days of the Pandemic in a Brazilian Public Hospital. Front Med. 9 de fevereiro de 2021; 8: 622577. [ Links ]
36.Ng JH, Hirsch JS, Hazzan A, Wanchoo R, Shah HH, Malieckal DA, et al. Outcomes Among Patients Hospitalized With COVID-19 and Acute Kidney Injury. American Journal of Kidney Diseases. Fevereiro de 2021; 77(2): 204-215. [ Links ]
37.Paek JH, Kim Y, Park WY, Jin K, Hyun M, Lee JY, et al. Severe acute kidney injury in COVID-19 patients is associated with in-hospital mortality. PLoS ONE. 9 de dezembro de 2020; 15(12): e0243528. [ Links ]
38.Li Q, Hu P, Kang H, Zhou F. Clinical Characteristics and Short-Term Outcomes of Acute Kidney Injury Missed Diagnosis in Older Patients with Severe COVID-19 in Intensive Care Unit. J Nutr Health Aging. Abril de 2021; 25(4): 492-500. [ Links ]
39.Doher MP, Torres de Carvalho FR, Scherer PF, Matsui TN, Ammirati AL, Caldin da Silva B, et al. Acute Kidney Injury and Renal Replacement Therapy in Critically Ill COVID-19 Patients: Risk Factors and Outcomes: A Single-Center Experience in Brazil. Blood Purif. 2021; 50(4-5):520-30. [ Links ]
40.Peng S, Wang H-Y, Sun X, Li P, Ye Z, Li Q, et al. Early versus late acute kidney injury among patients with COVID-19-a multicenter study from Wuhan, China. Nephrology Dialysis Transplantation. 4 de dezembro de 2020; 35(12): 2095-102. [ Links ]
41.Sang L, Chen S, Zheng X, Guan W, Zhang Z, Liang W, et al. The incidence, risk factors and prognosis of acute kidney injury in severe and critically ill patients with COVID-19 in mainland China: a retrospective study. BMC Pulm Med. Dezembro de 2020; 20(1): 290. [ Links ]
42.Su H, Yang M, Wan C, Yi L-X, Tang F, Zhu H-Y, et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney International. Julho de 2020; 98(1): 219-27. [ Links ]
43.Chen L, Li X, Chen M, Feng Y, Xiong C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovascular Research. 1º de Maio de 2020; 116(6): 1097-100. DOI: 10.1093/cvr/cvaa078 [ Links ]
44.Canatan D, Vives Corrons JL, De Sanctis V. The Multifacets of COVID-19 in Adult Patients: A Concise Clinical Review on Pulmonary and Extrapulmonary Manifestations for Healthcare Physicians: Covid-19 and Pulmonary and Extrapulmonary Manifestations. Acta Bio Medica Atenei Parmensis. 10 de novembro de 2020; 91(4): e2020173. DOI: 10.23750/abm.v91i4.10665 [ Links ]
45.Kolhe NV, Fluck RJ, Selby NM, Taal MW. Acute kidney injury associated with COVID-19: A retrospective cohort study. Remuzzi G, organizador. PLoS Med. 30 de outubro de 2020; 17(10): e1003406 [ Links ]
46.KIDIGO (Kidney Disease Improving Global Outcomes). KDIGO Clinical Practice Guideline for Acute Kidney Injury. In Kidney International Supplements; 2012; 1(2): 8-12. [ Links ]
47.Ministério da Saúde (BR). Manual de Apoio aos Gestores do SUS: Organização da Rede de Laboratórios Clínicos. 2003. 9 p. (Normas e Manuais Técnicos). [ Links ]
48.Iba T, Levy JH, Levi M, Thachil J. Coagulopathy in COVID-19. J Thromb Haemost. Setembro de 2020; 18(9): 2103-9. [ Links ]
49.Bhaskar S, Sinha A, Banach M, Mittoo S, Weissert R, Kass JS, et al. Cytokine Storm in COVID-19-Immunopathological Mechanisms, Clinical Considerations, and Therapeutic Approaches: The REPROGRAM Consortium Position Paper. Front Immunol. 10 de julho de 2020; 11: 1648. DOI: 10.3389/fimmu.2020.01648 [ Links ]
50.Parker K, Hamilton P, Hanumapura P, Castelino L, Murphy M, Challiner R, et al. Chronic anticoagulation is not associated with a reduced risk of acute kidney injury in hospitalised Covid-19 patients. BMC Nephrol. 2021; 22(1): 224. DOI: 10.1186/s12882-021-02436-5 [ Links ]
51.Oliveira IB. Bioquímica da Interação do SARS-CoV-2 com a Proteína ACE2 e Agravo da COVID-19 [Internet]. 2020. Avaliable from: https://editorarealize.com.br/editora/ebooks/conbracis/2020/trabalho_ev135_md7_sa100_id267_13112020154733.pdf [ Links ]
52.Borges KNG, Oliveira RC, Macedo DAP, Santos JC, Pellizzer LGM. O impacto da pandemia de COVID-19 em indivíduos com doenças crônicas e a sua correlação com o acesso a serviços de saúde. Rev Cient Esc Estadual Saúde Pública Goiás "Candido Santiago". 2020; 6(3): e6000013. [ Links ]
53.Macena WG, Hermano LO, Costa TC. Alterações fisiológicas decorrentes do envelhecimento. RM. 10 de maio de 2018; (27): 223-38. [ Links ]
54.Pimentel RMM, Daboin BEG, Oliveira AG de, Macedo Jr H. The dissemination of COVID-19: an expectant and preventive role in global health. J Hum Growth Dev. 27 de março de 2020; 30(1): 135-40. DOI: 10.7322/jhgd.v30.9976 [ Links ]
55.See YP, Young BE, Ang LW, Ooi XY, Chan CP, Looi WL, et al. Risk Factors for Development of Acute Kidney Injury in COVID-19 Patients: A Retrospective Observational Cohort Study. Nephron. 2021; 145(3): 256-64. DOI: 10.1159/000514064 [ Links ]
56.Ministério da Saúde (BR). Cartilha de Saúde dos Homens: como os Serviços de Saúde veem os Homens e o que podem tentar para cuidar deles [Internet]. Sociedade Brasileira de Medicina de Família e Comunidade (SBMFC); 2019. Avaliable from: https://www.sbmfc.org.br/wp-content/uploads/2019/12/cartilha-de-sau%cc%81de-do-homem.pdf [ Links ]
57.Kirby T. Evidence mounts on the disproportionate effect of COVID-19 on ethnic minorities. The Lancet Respiratory Medicine. junho de 2020;8(6):547-8. [ Links ]
58.Pan X, Xu D, Zhang H, Zhou W, Wang L, Cui X. Identification of a potential mechanism of acute kidney injury during the COVID-19 outbreak: a study based on single-cell transcriptome analysis. Intensive Care Med. junho de 2020; 46(6): 1114-6. DOI: 10.1007/s00134-020-06026-1 1 [ Links ]
59.Silva CMS e, Andrade AN, Nepomuceno B, Xavier DS, Lima E, Gonzalez I, et al. Evidence-based Physiotherapy and Functionality in Adult and Pediatric patients with COVID-19. J Hum Growth Dev. 2020; 30(1): 148-55. DOI: 10.7322/jhgd.v30.10086 1 [ Links ]
 Correspondence:
Correspondence:
jennifermarchiori49@gmail.com
Manuscript received: august 2021
Manuscript accepted: september 2021
Version of record online: november 2021