Servicios Personalizados
Revista
Articulo
Indicadores
Compartir
Aletheia
versión impresa ISSN 1413-0394
Aletheia vol.49 no.1 Canoas ene./jun. 2016
INTERNATIONAL ARTICLES
Post-traumatic stress disorder and substance use following traumatic brain injury: a clinical and neuropsychological case study
Transtorno de estresse pós-traumático e uso de substância após traumatismo crânio-encefálico: um estudo de caso clínico e neuropsicológico
Saulo Gantes Tractenberg1, I; Bruno Kluwe-Schiavon2, II; Rodrigo Orso3, I; Gabriela dos Santos Jacobsen4, I; Julio Carlos Pezzi5, III; Rodrigo Grassi-Oliveira6, I, IV
IPontifícia Universidade Católica do Rio Grande do Sul (PUCRS), Porto Alegre, Brazil
IIUniversity of Zurich, Zurich, Switzerland
IIISistema de Saúde Mãe de Deus, Porto Alegre, Brazil
IVBrain Institute (InsCer), Porto Alegre, Brazil
ABSTRACT
The present study proposes to investigate the case of a patient with crack-cocaine use disorder from the occurrence of a neurological condition of traumatic brain injury (TBI) and development of Post-Traumatic Stress Disorder (PTSD). It is presumed that this is a case of crack and cocaine use disorder from the occurrence of two predisposing factors: TBI and the appearance of post-traumatic symptoms. Therefore, the present case discusses, clinically and based in neuropsychological assessment, the hypotheses of substance use as self-medication to attenuate the depressive symptoms related to the traumatic experience and/or as a consequence of a neuropsychological framework. Furthermore, the presence of a neurological condition may explain the subsequent progression to crack-cocaine use disorder.
Keywords: Crack/cocaine, Traumatic brain injury, Case study.
RESUMO
O presente estudo se propôs a investigar o caso de uma paciente dependente de cocaína e crack, a qual desenvolveu o quadro de dependência após ter sido diagnosticada com Traumatismo Crânio-Encefálico (TCE) em decorrência de um acidente e Transtorno de Estresse Pós-Traumático. Este caso, em especial, por apresentar co-ocorrência de condições neurológicas e psiquiátricas foi alvo de uma avaliação clínica e neuropsicológica. A hipótese do uso de substância como forma de automedicação pode estar relacionada com o início e progressão para dependência de cocaína e crack, uma vez que foram reportados sintomas depressivos e pós-traumáticos acentuados. Além disso, a presença de um quadro neurológico com possíveis alterações neuropsicológicas associadas pode explicar a subsequente progressão para dependência de cocaína e crack.
Palavras-chave: Crack, Traumatismo crânio-encefálico, Estudo de caso.
Introduction
Traumatic Brain Injury (TBI) has been associated with the development of several psychiatric disorders, including Mood Disorder (MD), Post-traumatic Stress Disorder PTSD), and Substance Use Disorder (SUD) (Ashman et al., 2004; Fann et al., 2004). It is estimated that 10% to 30% of individuals with TBI are also diagnosed with PTSD, and the rate may be higher among individuals who had brief or extended periods of unconsciousness due to the trauma. Related to SUD, there is evidence indicating an association between certain patterns of consumption and TBI history (Hoffman, Dikmen, Temkin, & Bell, 2012). Addictive patients who have experienced TBI, for example, revealed an increased risk of re-injury and suicide attempts, as well as a reduced life satisfaction rates and poor clinical outcomes (Bogner & Corrigan, 2013). Further, some evidences support that TBI represents a risk factor for SUD (Bogner & Corrigan, 2013; Taylor, Kreutzer, Demm, & Meade, 2003b). Despite TBI has been commonly associated with PTSD and SUD, corroborating to negative prognosis and functional outcomes (Brady et al., 2009; Vasterling & Dikmen, 2012), few studies addressed whether PTSD and/or SUD will be able to affect the functional and neurocognitive profile following TBI.
TBI patients – depending on the type (open or closed) and the severity of the injury – have a wide array of neuropsychological impairments (e.g. attention, memory, speed of information processing, planning and problem resolution, inhibitory control and emotional processing) (Frencham, Fox, & Maybery, 2005; Milders, Fuchs, & Crawford, 2003) and behavioral dysfunction (e.g. impaired inhibitory control, impaired emotional self-regulation and impulsivity) (Kennedy et al., 2007; Vasterling & Dikmen, 2012). Interestingly, a similar neuropsychological profile can also be observed in individuals with SUD (Koso & Hansen, 2006; Suliman, Troeman, Stein, & Seedat, 2014), suggesting that the co-occurrence of TBI and SUD could exacerbate these neuropsychological impairments, leading to a worse prognosis (Kolakowsky-Hayner & Kreutzer, 2001; Sander et al., 2012). Therefore, studies have suggested that substance use predicts increased disability and delayed recovery in TBI patients, and individuals with TBI and co-occurring SUD have been shown to have poorer neurocognitive performance and behavioral functioning (Corrigan, 1995). Furthermore, there is evidence that co-occurrence of PTSD and TBI is common, especially among war veterans and several possible explanation have already emerged (Bryant, 2011). These explanations are focused on the anterograde and retrograde amnesia since TBI may affect the individual's capacity to contextualize the experience in autobiographical memory (Bryant, 2011; ; Piolino et al., 2007). In the same way, it appears that the development of PTSD is also influenced by the characteristics of TBI injury, as well as some personality traits.
Finally, despite the co-occurrence between PTSD or SUD and TBI, there is also a well-known association between PTSD and SUD (Back, Waldrop, & Brady, 2009; Eiroa-Orosa, Giannoni-Pastor, Fidel-Kinori, & Arguello, 2016). Some authors suggest that SUD may represent a self-medication attempt to avoid PTSD symptoms (Eiroa-Orosa et al., 2016; Sheerin et al., 2016). Moreover, PTSD symptoms are strongly correlated with increased craving and high relapse rates in SUD. As mentioned, co-occurring psychiatric conditions are one of the major clinical challenges due to the variety of treatment intercurrences. So a complex interaction between neurological disorder (TBI) and psychiatric conditions (PTSD and/or SUD) could aggravate the prognosis and the course of rehabilitation treatments. For this reason, the aim of this case study was to investigate the complex neuropsychiatric framework of TBI followed by PTSD and SUD(crack-cocaine use disorder) by a neuropsychological perspective.
Case description
This article describes the case of a 39 years old crack-cocaine dependent woman (F), inpatient of a detoxification public unit in Porto Alegre, Brazil, who had previously suffered a TBI and subsequently developed PTSD and SUD. F. was born in São Paulo, Brazil, and referred 16 years of formal education, including a PhD in Philosophy and fluency in five foreign languages. According to the psychiatrist responsible for the unit, F. had lack of impulse /control and emotional regulation, demonstrating difficulties in craving management and severe withdrawal and PTSD symptoms during detoxification treatment. Additionally, F. constantly reported difficulties with activities that require attention, such as reading a book or attending group therapy. A clinical and neuropsychological evaluation was required by the psychiatrist and it was conducted by a psychologist for research proposals.
History
F. used cocaine for the first time in 2009 after she was discharged from a hospital where she had spent one month in coma due to a car accident that has resulted in multiple fractures and severe TBI. Her partner (with whom she had been in a stable relationship for 12 years) died as consequence of the accident. F. reported that she remembers details of the moments that preceded and followed the accident and claimed to have witnessed the death of her partner.
When she was asked about the substance use, F. reported that she rarely drank during the period prior to the accident, and that she only started sporadic use of cocaine few days after being discharged from the hospital to "avoid feeling emotions". Over the next two years F. gradually began to use cocaine with higher frequency, first using a few times per week (often at the weekend) with some withdrawal periods. However, during 2010 and 2011, one year after she had started the cocaine use, her consumption pattern leaded to considerable distress and impairments on professional and social activities. During 2011 F. used crack for the first time in a one-week binge episode. Afterwards, F. lost her job and had to sell personal belongings to obtain the drug, being judicially interdicted by her relatives. When she was asked about her current crack-cocaine use consumption pattern, she reported to be a chronic user for 4 years, using up to 5-6 grams of crack per day. Additionally, F. also reported that her longest abstinence period in the last three years was during her current hospitalization (25 days).
Procedures and instruments
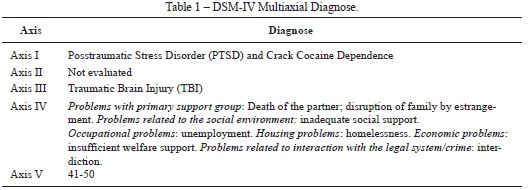
The clinical and neuropsychological assessment took place during two interviews lasting approximately 90 minutes each, on the eighteenth and twentieth days of hospitalization following her admission to the treatment program. The standard treatment program included a cocaine detoxification protocol, antidepressants, neuroleptics, analgesics and mood stabilizers, if necessary. No benzodiazepines or other sedatives were administered in the 24 hours before the clinical and neuropsychological assessment. The first interview focused on her social history (e.g. occupation, marital status, family), past medical history (TBI) and as well as the history of the present illness (SUD and PTSD). The Structured Clinical Interview for DSM-IV-TR Disorders (SCID-I) was used to investigate past and current diagnosis (Del-Ben et al., 2001). The Beck Depression Inventory (BDI-II) (Gomes-Oliveira, Gorenstein, Lotufo Neto, Andrade, & Wang, 2012) and the Cocaine Selective Severity Assessment (CSSA) were used as supplementary clinical assessments to access depressive and craving/withdrawal symptoms (Kampman et al., 1998). The CSSA version used was adapted later to crack/cocaine by Kluwe- Schiavon and colleagues (2015). The second interview consisted of a neuropsychological assessment. The instruments used are described briefly in Table 1.
Data analysis and synthesis of results
Regarding the clinical assessment, the SCID-I confirmed that F. met the criteria for current PTSD and Crack Cocaine Dependence (based on DSM-IV diagnosis criteria). F. also had a previous diagnosis of TBI following a car accident. Because we had a short time for evaluation, we did not investigate personality disorders. Considering the multiaxial division, we reported F. psychosocial and legal problems and her global assessment of functioning (GAF) classification (for detailed DSM-IV multiaxial diagnose, check table X). The clinical symptoms evaluation of F. revealed that she had moderate depressive and craving symptoms assessed by both BDI and CSSA, respectively.
Concerning the neuropsychological assessment, F. obtained an estimate IQ of 91 confidence interval of 95%, QI = 85-98), suggesting an IQ between 'below average' and 'average' cutoffs (Yates et al., 2006). For the neuropsychological tests the Z-scores (score of the case minus the average of the reference group, divided by the standard deviation of the reference group) were calculated and a threshold of Z < -1.5 was used as the criterion for neuropsychological impairment (Kave, Heled, Vakil, & Agranov, 2011) (see Table 2).
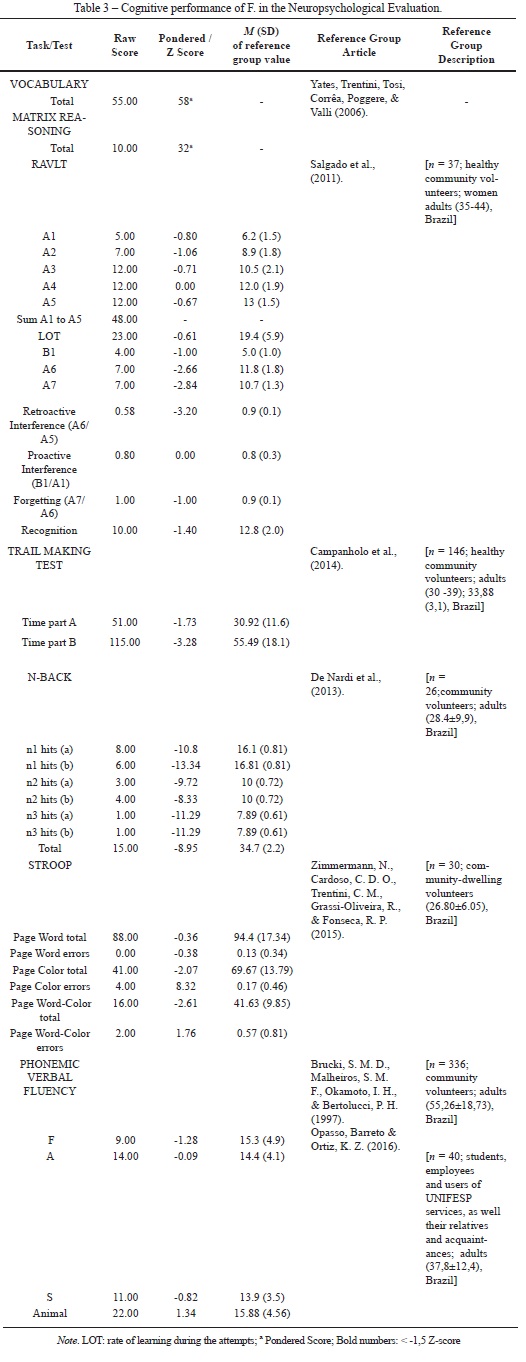
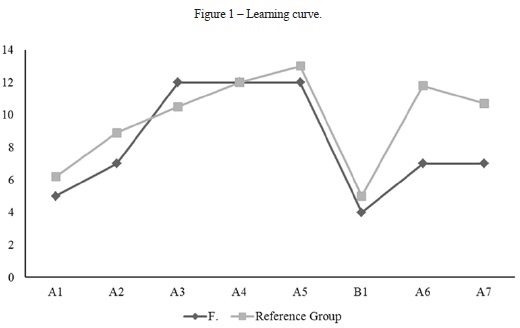
Regarding memory, the scores of RAVLT suggest that F. has deficits in immediate memory recall, experiencing problems with retroactive interference and delayed memory recall (Figure 1, see learning curve), but no learning impairment. This finding suggests that her capacity to learn across trials was preserved, but she showed some impairment in storing the information due to the interference of external stimuli upon the encoded information.
The results of the N-Back task suggest that F. has a working memory impairment. As observed in Table 2, the overall performance on the N-Back task was poor (particularly on the 'back 2' and 'back 3' conditions) in comparison with a reference group of healthy adults. However, F's performance was in line with the mean performance of a sample of crack cocaine users tested previously by our research group (Figure 1, reference group), although she had more years of education (16 years) than the reffered crack cocaine sample (M = 8.72 years of education, SD = 2.78). Additionally, the large number of intermittent errors observed in the N-Back task compared to the lack of persistent errors also reinforces the hypothesis of an attentional impairment. The findings on attention and memory tasks suggest that although F has some difficulties with focused attention and immediate recall, she can store information and retrieve it when using environmental cues semantically related to this information.
Regarding executive functions, the Stroop Color Interference Test revealed a deficit in inhibitory control. F made four errors on the color page and two errors on the word-color page. Her net score (16) was in the 90th percentile, representing above-average performance for both TBI patients and healthy adults. The results of the TMT showed that F took 51s to complete TMT A and 115s to complete TMT B, making error in the two parts of the test (one error in TMT A and two errors in TMT B, respectively). These parameters in TMT suggest that F has deficits in processing speed and cognitive flexibility. Further, F. committed error in both parts of the test, which may suggest deficits in sustained attention and attentional switching.
Discussion
This study investigated the interaction of neuropsychiatric conditions, in which a TBI was succeeded by PSTD and SUD. A clinical and neuropsychological profile was constructed to obtain a better understanding of how the patient issues were affected by the co-occurrence of multiple disorders that are widely recognized to have a deleterious effect on clinical condition and cognitive functioning, respectively. This case has some particularities that should be highlighted. The patient profile, her history and pattern of drug use are distinct from most of other patients admitted on public detoxification units and, for this reason, justify a special attention. For example, F. has a high level of education, which is not common between crack cocaine inpatients in this type of unit. Furthermore, there was a late onset of crack cocaine use (cocaine onset at 35's and crack onset at 37's) and the how it began, after a traumatic experience in which F. suffered a TBI with conscience loss.
The psychiatry condition of F. (current PTSD and Crack Cocaine Dependence; past diagnosis of TBI), highlights the importance for clinicians to differentiate the co-occurrence of multiples diagnosis from cases in which one disorder is induced by another. In the clinical history of F. it was clear that after a car accident (in which she suffered a TBI), she developed many symptoms specifically related to the trauma and not necessarily to her neurological condition. These symptoms were persistent and did not remit in the first month, progressing to a PTSD diagnosis. Furthermore, despite the reasons that corroborated to F onset of substance use were not fully understood, she developed a pattern of cocaine and crack use in the subsequent years, supporting SUD diagnosis. She had severe functional and psychosocial impairments that culminate to her hospitalization. In this sense, this clinical rational suggested the presence, independently, of all three diagnoses, not ignoring the possibility of a relationship in their course according F's clinical history. In addition, despite our evaluation was based on DSM-IV diagnosis criteria, we believed that it remains unchanged even if we consider the DSM-V criteria. For example, in PTSD diagnosis, F. already presented symptoms of negative alteration of cognition and mood (e.g., numbing symptoms and cognitive distortion about the cause and consequences of the trauma), which comprehend the new cluster criteria (D criteria). Related to Substance Use Diagnosis, in which DSM-V did not distinguish anymore abuse from dependence, the appropriate nomenclature for F. case would be replaced by Crack Cocaine Use Disorder.
The clinical assessment of F. revealed severe craving (maximum score on questions 4 and 5 of CSSA) and symptoms of depression (total score on BDI-II indicating moderate to severe depression), even if the assessment was conducted towards the end of hospitalization, at that point it was expected a significant reduction in depression and withdrawal symptoms (Francke, Viola, Tractenberg, & Grassi-Oliveira, 2013; Viola et al., 2013). These patterns of clinical profile are similar than found by Francke and colleagues (2013) in a sample of crack cocaine dependent women who experienced trauma early in life. Although F. does not present trauma in her early history, the presence of trauma history and PTSD diagnosis could contribute to the higher depressive and withdrawal symptomatology. Regarding PTSD symptoms, the patient also reported almost all the symptoms listed in the DSM-IV criteria for PTSD. She referred symptoms of reexperiencing the event through mental images, avoidance of all cues associated with the trauma and symptoms of emotional numbness. This pattern of symptoms is consistent with previous reports that PTSD symptoms (e.g. derealization, guilt, emotional numbing) are more severe in patients with PTSD and SUD, suggesting that patients with comorbidity of PTSD and SUD are more likely to experience psychosocial problems than patients with PTSD alone (Narvaez et al., 2014; Salgado, Quinlan, & Zlotnick, 2007).
In the neuropsychological evaluation, we observed some dysfunctions that reinforce the comprehension of progression to SUD following both TBI and trauma. For example, in the Stroop Test, the impaired performance of F. in page-color and page word-color revealed an inhibitory control deficit. Inhibitory deficits are known as related to development of dysfunctional behavioral tendencies including impulsivity, aggression and a propensity for risky behaviors in individuals with TBI (Graham & Cardon, 2008; Teasdale & Engberg, 2001). These behaviors are commonly associated with an increase vulnerability mediating the transitions between SUD stages (Graham & Cardon, 2008; West, 2011). Other behavioral deficits, including difficulty in emotional regulation, depressive and anxiety behaviors, apathy and mood instability are also considered risk factors for drug addiction in TBI patients (Graham & Cardon, 2008; Taylor, Kreutzer, Demm, & Meade, 2003a) and were reported by the psychiatrist or observed in clinical profile of the patient described above. Given that these behavioral symptoms could be related or may be a consequence of cognitive flexibility impairments and inhibitory dysfunction, it is possible that progression from TBI to SUD is the result of interaction of a specific cognitive and behavior profile commonly observed in patients following severe TBI with PTSD.
In addition, others neuropsychological alterations were found, suggesting deficits in attention, immediate recall, working memory and cognitive flexibility. These findings are in accordance with a study by Jong, Zafonte, Millis & Yavuzer (1999) which found that individuals with TBI and co-occurring cocaine abuse showed poor performance on learning and memory tasks compared to individuals with only TBI. Memory is a multi-stage process (encoding, consolidation, storage and retrieval) and Vanderploeg and colleagues (2014) suggested that specific memory processes, mainly encoding and consolidation, could be particularly affected by TBI. Although F. seem to have preserved both encoding and learning capacities (see trial 1 and learning curve data), she revealed a significant impairment in both immediate and delayed recall. These findings support that consolidation and retrieval memory processes could be affected by her drug addiction, since this neuropsychological profile has been previously found in cocaine and crack cocaine users (Viola et al., 2015).
Although memory impairments are often a consequence of TBI, some authors have argued that a general deficit in executive functioning is behind the memory impairments. It has been suggested that the poor performance of individuals with TBI on free recall tasks may be an indicative of executive dysfunction (O'Brien, Chiaravalloti, Arango-Lasprilla, Lengenfelder, & DeLuca, 2007; Vanderploeg et al., 2014). This hypothesis receives some support from F's performance on executive functioning tasks; F showed poor performance on almost all the executive function tasks tested (TMT A and TMT B, Stroop Test and FAS), suggesting that her difficulties in retrieving and selecting effective problem-solving strategies might explain her poor performance on the free recall tasks(Vanderploeg et al., 2014), and thus contribute to the cognitive and behavioral difficulties she described.
In view of the findings from the clinical and neuropsychological assessment, we believe that none of the conditions from which F suffered could be solely responsible for her cognitive impairments. We consider that the co-occurrence of TBI, PTSD and SUD may have increased the magnitude of the functional deficits she displayed, as interactions among multiple clinical conditions are associated with an aggravation of cognitive deficits. Based on the chronology of the patient's clinical history (TBI followed by PTSD followed by SUD), the symptom profile (PTSD symptoms and severe craving symptoms) and the patient's account of her history and problems, we hypothesize that the onset of crack cocaine use may be attributable to an attempt at self-medication. The history of post-traumatic symptoms and pattern of crack cocaine usage described by the patient also corroborates the self-medication hypothesis (Back et al., 2009; Brady et al., 2009), suggesting that substance use was motivated by a desired to reduce the emotional distress associated with the traumatic experience. A prior study of Tractenberg and colleagues (2012) indicated that could be an association between the traumatic experience and the onset of crack cocaine use. In general, the first use of crack/cocaine tends to be after a traumatic experience (Johnson, Striley, & Cottler, 2006). Since both snorted and smoked crack cocaine have similar pharmacological properties, it is possible that both could be used for F's self-medication. Although self-medication hypothesis has more evidences with drugs that have depressant and anxiolytic effects, such as alcohol and benzodiazepines, the study of Waldrop and collegues (2007) suggested that use of crack cocaine over a short period might reduce emotional symptoms (Waldrop, Back, Verduin, & Brady, 2007). On the other hand, chronic crack/cocaine use has been suggested as associated to an intensification of posttraumatic symptoms (Back, Brady, Jaanimägi, & Jackson, 2006; Bremner, Southwick, Darnell, & Charney, 1996), which suggests that self-medication would be counterproductive. Overall it seems that the effect of crack cocaine use on PTSD symptoms is time-dependent. The difficulties F. experienced during detoxification and the lack of attenuation of her PTSD symptoms may have been due to her chronic use of crack cocaine. Finally, it is difficult to confirm any neuropsychological hypothesis since we did not have any information or data about her pre-morbid condition on her cognitive functioning.
An understanding of the course of each disorder and how the clinical and neuropsychological symptoms interact during rehabilitation is essential to providing an accurate prognosis and planning an appropriate treatment. In the case of F, the history suggests that an intervention targeting her PTSD symptoms, such as trauma focused cognitive-behavioral therapy – an evidence-based treatment to help individuals manage and modify negative emotional responses to a traumatic event – should be considered as an adjunct to the treatment for crack cocaine use disorder. There is a consensus in the literature on comorbid PTSD and substance use that improvement of PTSD symptoms is associated with a reduction in withdrawal symptoms when integrated interventions are used (Brady, Dansky, Back, Foa, & Carroll, 2001; Hien et al., 2010). In cases where TBI is complicated by comorbid substance use neuropsychological rehabilitation should only be considered after a prolonged period of abstinence.
References
Ashman, T. A., Spielman, L. A., Hibbard, M. R., Silver, J. M., Chandna, T., & Gordon, W. A. (2004). Psychiatric challenges in the first 6 years after traumatic brain injury: crosssequential analyses of Axis I disorders. Arch Phys Med Rehabil, 85(2), 36-42. [ Links ]
Back, S. E., Brady, K. T., Jaanimägi, U., & Jackson, J. L. (2006). Cocaine dependence and PTSD: a pilot study of symptom interplay and treatment preferences. Addict Behav, 31(2), 351-354. [ Links ]
Back, S. E., Waldrop, A. E., & Brady, K. T. (2009). Treatment challenges associated with comorbid substance use and posttraumatic stress disorder: clinicians' perspectives. Am J Addict, 18(1), 15-20. [ Links ]
Bogner, J., & Corrigan, J. D. (2013). Interventions for Substance Misuse following TBI: A Systematic Review. Brain Impairment, 14(1), 77-91. [ Links ]
Brady, K. T., Dansky, B. S., Back, S. E., Foa, E. B., & Carroll, K. M. (2001). Exposure therapy in the treatment of PTSD among cocaine-dependent individuals: preliminary findings. J Subst Abuse Treat, 21(1), 47-54. [ Links ]
Brady, K. T., Tuerk, P., Back, S. E., Saladin, M. E., Waldrop, A. E., & Myrick, H. (2009). Combat posttraumatic stress disorder, substance use disorders, and traumatic brain injury. J Addict Med, 3;(4), 179-188. [ Links ]
Bremner, J. D., Southwick, S. M., Darnell, A., & Charney, D. S. (1996). Chronic PTSD in Vietnam combat veterans: course of illness and substance abuse. Am J Psychiatry, 153(3), 369-375. [ Links ]
Bryant, R. (2011). Post-traumatic stress disorder vs traumatic brain injury. Dialogues Clin Neurosci, 13(3), 251-262. [ Links ]
Campanholo, K. R., Romão, M. A., Machado, M. d. A. R., Serrao, V. T., Coutinho, D. G. C., Benute, G. R. G., & Lucia, M. C. S. d. (2014). Performance of an adult Brazilian sample on the Trail Making Test and Stroop Test. Dementia & Neuropsychologia, 8, 26-31. [ Links ]
Corrigan, J. D. (1995). Substance abuse as a mediating factor in outcome from traumatic brain injury. Arch Phys Med Rehabil, 76(4), 302-309. [ Links ]
De Nardi, T., Sanvicente-Vieira, B., Prando, M., Stein, L. M., Fonseca, R. P., & Grassi-Oliveira, R. (2013). Tarefa N-Back Auditiva: Desempenho entre Diferentes Grupos Etários. Psicologia: Reflexão e Crítica, 26(1), 8. [ Links ]
Del-Ben, C. M., Vilela, J. A. A., Crippa, J. A. d. S., Hallak, J. E. C., Labate, C. M., & Zuardi, A. W. (2001). Confiabilidade da "Entrevista Clínica Estruturada para o DSMIV – Versão Clínica" traduzida para o português. Rev Bras Psiquiatr, 23, 156-159.
Eiroa-Orosa, F. J., Giannoni-Pastor, A., Fidel-Kinori, S. G., & Arguello, J. M. (2016). Substance use and misuse in burn patients: Testing the classical hypotheses of the interaction between post-traumatic symptomatology and substance use. J Addict Dis, 35(3), 194-204. [ Links ]
Fann, J. R., Burington, B., Leonetti, A., Jaffe, K., Katon, W. J., & Thompson, R. S. (2004). Psychiatric illness following traumatic brain injury in an adult health maintenance organization population. Arch Gen Psychiatry, 61(1), 53-61. [ Links ]
Francke, I. D., Viola, T. W., Tractenberg, S. G., & Grassi-Oliveira, R. (2013). Childhood neglect and increased withdrawal and depressive severity in crack cocaine users during early abstinence. Child Abuse Negl, 37(10), 883-889. [ Links ]
Frencham, K. A., Fox, A. M., & Maybery, M. T. (2005). Neuropsychological studies of mild traumatic brain injury: a meta-analytic review of research since 1995. J Clin Exp Neuropsychol, 27(3), 334-351. [ Links ]
Gomes-Oliveira, M. H., Gorenstein, C., Lotufo Neto, F., Andrade, L. H., & Wang, Y. P. (2012). Validation of the Brazilian Portuguese version of the Beck Depression Inventory-II in a community sample. Rev Bras Psiquiatr, 34(4), 389-394. [ Links ]
Hien, D. A., Jiang, H., Campbell, A. N., Hu, M. C., Miele, G. M., Cohen, L. R., . . . Nunes, E. V. (2010). Do treatment improvements in PTSD severity affect substance use outcomes? A secondary analysis from a randomized clinical trial in NIDA's Clinical Trials Network. Am J Psychiatry, 167(1), 95-101. [ Links ]
Hoffman, J. M., Dikmen, S., Temkin, N., & Bell, K. R. (2012). Development of posttraumatic stress disorder after mild traumatic brain injury. Arch Phys Med Rehabil, 93(2), 287-292. [ Links ]
Johnson, S. D., Striley, C., & Cottler, L. B. (2006). The association of substance use disorders with trauma exposure and PTSD among African American drug users. Addict Behav, 31(11), 2063-2073. [ Links ]
Jong, C. N., Zafonte, R. D., Millis, S. R., & Yavuzer, G. (1999). The effect of cocaine on traumatic brain injury outcome: a preliminary evaluation. Brain Inj, 13(12), 1017-1023. [ Links ]
Kampman, K. M., Volpicelli, J. R., McGinnis, D. E., Alterman, A. I., Weinrieb, R. M., D'Angelo, L., & Epperson, L. E. (1998). Reliability and validity of the Cocaine Selective Severity Assessment. Addict Behav, 23(4), 449-461. [ Links ]
Kave, G., Heled, E., Vakil, E., & Agranov, E. (2011). Which verbal fluency measure is most useful in demonstrating executive deficits after traumatic brain injury? Journal of Clinical and Experimental Neuropsychology, 33(3), 358-365. [ Links ]
Kennedy, J. E., Jaffee, M. S., Leskin, G. A., Stokes, J. W., Leal, F. O., & Fitzpatrick, P. J. (2007). Posttraumatic stress disorder and posttraumatic stress disorder-like symptoms and mild traumatic brain injury. J Rehabil Res Dev, 44(7), 895-920. [ Links ]
Kluwe-Schiavon, B., Tractenberg, S. G., Sanvicente-Vieira, B., Rosa, C. S. d. O., Arteche, A. X., Pezzi, J. l. C., & Grassi-Oliveira, R. (2015). Propriedades psicométricas da Cocaine Selective Severity Assessment (CSSA) em mulheres usuárias de crack. Jornal Brasileiro de Psiquiatria, 64, 115-121. [ Links ]
Kolakowsky-Hayner, S. A., & Kreutzer, J. S. (2001). Pre-injury crime, substance abuse, and neurobehavioural functioning after traumatic brain injury. Brain Injury, 15(1), 53-63. [ Links ]
Koso, M., & Hansen, S. (2006). Executive function and memory in posttraumatic stress disorder: a study of Bosnian war veterans. Eur Psychiatry, 21(3), 167-173. [ Links ]
Milders, M., Fuchs, S., & Crawford, J. R. (2003). Neuropsychological impairments and changes in emotional and social behaviour following severe traumatic brain injury. J Clin Exp Neuropsychol, 25(2), 157-172. [ Links ]
Narvaez, J. C., Jansen, K., Pinheiro, R. T., Kapczinski, F., Silva, R. A., Pechansky, F., & Magalhães, P. V. (2014). Psychiatric and substance-use comorbidities associated with lifetime crack cocaine use in young adults in the general population. Compr Psychiatry. [ Links ]
O'Brien, A., Chiaravalloti, N., Arango-Lasprilla, J. C., Lengenfelder, J., & DeLuca, J. (2007). An investigation of the differential effect of self-generation to improve learning and memory in multiple sclerosis and traumatic brain injury. Neuropsychol Rehabil, 17(3), 273-292. [ Links ]
Opasso, P. R., Barreto, S. d. S., & Ortiz, K. Z. (2016). Phonemic verbal fluency task in adults with high-level literacy. Einstein (São Paulo), 14, 398-402. [ Links ]
Piolino, P., Desgranges, B., Manning, L., North, P., Jokic, C., & Eustache, F. (2007). Autobiographical memory, the sense of recollection and executive functions after severe traumatic brain injury. Cortex, 43(2), 176-195. [ Links ]
Salgado, D. M., Quinlan, K. J., & Zlotnick, C. (2007). The relationship of lifetime polysubstance dependence to trauma exposure, symptomatology, and psychosocial functioning in incarcerated women with comorbid PTSD and substance use disorder. J Trauma Dissociation, 8(2), 9-26. [ Links ]
Salgado, J. V., Malloy-Diniz, L. F., Abrantes, S. S. C., Moreira, L., Schlottfeldt, C. G., Guimarães, W., Freitas, D. M. U., Oliveira, J. & Fuentes, D. (2011). Applicability of the Rey Auditory-Verbal Learning Test to an adult sample in Brazil. Revista Brasileira de Psiquiatria, 33(3), 3. [ Links ]
Sander, A. M., Bogner, J., Nick, T. G., Clark, A. N., Corrigan, J. D., & Rozzell, M. (2012). A Randomized Controlled Trial of Brief Intervention for Problem Alcohol Use in Persons With Traumatic Brain Injury. Journal of Head Trauma Rehabilitation, 27(5), 319-330. [ Links ]
Sheerin, C., Berenz, E. C., Knudsen, G. P., Reichborn-Kjennerud, T., Kendler, K. S., Aggen, S. H., & Amstadter, A. B. (2016). A population-based study of help seeking and self-medication among trauma-exposed individuals. Psychol Addict Behav, 30(7), 771-777. [ Links ]
Suliman, S., Troeman, Z., Stein, D. J., & Seedat, S. (2014). Are neuropsychological deficits after trauma associated with ASD severity? Compr Psychiatry, 55(1), 145-154. [ Links ]
Taylor, L. A., Kreutzer, J. S., Demm, S. R., & Meade, M. A. (2003a). Traumatic brain injury and substance abuse: A review and analysis of the literature. Neuropsychol Rehabil, 13(1-2), 165-188. [ Links ]
Taylor, L. A., Kreutzer, J. S., Demm, S. R., & Meade, M. A. (2003b). Traumatic brain injury and substance abuse: A review and analysis of the literature. Neuropsychological Rehabilitation, 13(1-2), 165-188. [ Links ]
Teasdale, T. W., & Engberg, A. W. (2001). Suicide after traumatic brain injury: a population study. J Neurol Neurosurg Psychiatry, 71(4), 436-440. [ Links ]
Tractenberg, S. G., Viola, T. W., Rosa, C. S. d. O., Donati, J. M., Francke, I. D. A., Pezzi, J. C., & Grassi-Oliveira, R. (2012). Exposição a trauma e transtorno de estresse póstraumático em usuárias de crack. Jornal Brasileiro de Psiquiatria, 61, 206-213. [ Links ]
Vanderploeg, R. D., Donnell, A. J., Belanger, H. G., & Curtiss, G. (2014). Consolidation deficits in traumatic brain injury: The core and residual verbal memory defect. J Clin Exp Neuropsychol, 36(1), 58-73. [ Links ]
Vasterling, J. J., & Dikmen, S. (2012). Mild traumatic brain injury and posttraumatic stress disorder: clinical and conceptual complexities. J Int Neuropsychol Soc, 18(3), 390-393. [ Links ]
Viola, T. W., Tractenberg, S. G., Kluwe-Schiavon, B., Levandowski, M. L., Sanvicente-Vieira, B., Wearick-Silva, L. E., . . . Grassi-Oliveira, R. (2015). Brain-Derived Neurotrophic Factor and Delayed Verbal Recall in Crack/Cocaine Dependents. Eur Addict Res, 21(5), 273-278. [ Links ]
Viola, T. W., Tractenberg, S. G., Levandowski, M. L., Levandowski, M. L., Pezzi, J. C., Bauer, M. E., . . . Grassi-Oliveira, R. (2013). Neurotrophic factors in women with crack cocaine dependence during early abstinence: the role of early life stress. J Psychiatry Neurosci, 38(6), 1300-27. [ Links ]
Waldrop, A. E., Back, S. E., Verduin, M. L., & Brady, K. T. (2007). Triggers for cocaine and alcohol use in the presence and absence of posttraumatic stress disorder. Addict Behav, 32(3), 634-639. [ Links ]
West, S. L. (2011). Substance use among persons with traumatic brain injury: a review. NeuroRehabilitation, 29(1), 1-8. [ Links ]
Yates, D. B., Trentini, C. M., Tosi, S. D., Corrêa, S. K., Poggere, L. C., & Valli, F. (2006). Apresentação da Escala de Inteligência Wechsler Abreviada (WASI). Avaliação Psicológica, 5(2), 6. [ Links ]
Zimmermann, N., Cardoso, C. d. O., Trentini, C. M., Grassi-Oliveira, R., & Fonseca, R. P. (2015). Brazilian preliminary norms and investigation of age and education effects on the Modified Wisconsin Card Sorting Test, Stroop Color and Word test and Digit Span test in adults. Dementia & Neuropsychologia, 9, 120-127. [ Links ]
 Correspondence to
Correspondence to
E-mail:saulo.tractenberg@acad.pucrs.br
Recebido em outubro de 2016
Aprovado em maio de 2017
1 Tractenberg, S.G.: Developmental Cognitive Neuroscience Lab (DCNL), Programa de Pós-graduação em Psicologia da Pontifícia Universidade Católica do Rio Grande do Sul (PUCRS), Porto Alegre, Brazil.
2 Kluwe-Schiavon, B.: Experimental and Clinical Pharmacopsychology Lab, University of Zurich, Zurich, Switzerland.
3 Orso, R.: DCNL, Programa de Pós-graduação em Pediatria e Saúde PUCRS, Porto Alegre, Brazil.
4 Jacobsen, G.S.: DCNL, Programa de Pós-graduação em Psicologia da PUCRS, Porto Alegre, Brazil.
5 Pezzi, J.C.: Sistema de Saúde Mãe de Deus, Porto Alegre, Brazil.
6 Grassi-Oliveira, R.: DCNL, Programa de Pós-graduação em Psicologia da PUCRS, Porto Alegre, Brazil. Brain Institute (InsCer), Porto Alegre, Brazil.