Serviços Personalizados
Journal
artigo
Indicadores
Compartilhar
Journal of Human Growth and Development
versão impressa ISSN 0104-1282versão On-line ISSN 2175-3598
J. Hum. Growth Dev. vol.31 no.2 Santo André maio/ago. 2021
https://doi.org/10.36311/jhgd.v31.12228
ORIGINAL ARTICLE
Use of psychotropic drugs in the treatment of fibromyalgia: a systematic review
Ana Vívian Ferreira da CostaI; Larissa de Carvalho BezerraII; Juliane dos Anjos de PaulaIII
IMédica pela Faculdade de Medicina Estácio de Juazeiro do Norte- FMJ,CE- Brasil;
IIAcadêmica do curso de Medicina da Faculdade de Medicina Estácio de Juazeiro do Norte- CE, Brasil;
IIIMestrado em ciências da saúde pela faculdade de Medicina do ABC, Residência Médica em Psiquiatria no Hospital das Clínicas da UFPE, Professora do curso de medicina na disciplina de saúde mental da Faculdade de Medicina Estácio de Juazeiro do Norte -FMJ, Brasil
ABSTRACT
INTRODUCTION: The treatment of fibromyalgia is evolving, and more and more drugs are available on the market
OBJECTIVE: To verify the response, tolerability, and adverse events of the use of psychotropic drugs in the treatment of fibromyalgia
METHODS: A systematic review of articles on fibromyalgia and psychotropic medications were carried out, indexed in the MEDLINE database (PUBMED) with the MeSH terms: "fibromyalgia", "psychotropic drugs," and "treatment outcome". Of the 89 studies identified, 23 met the eligibility criteria
RESULTS: It has been seen that some classes of psychotropic medications have significantly improved patients' painful episodes, which have an important positive impact on quality of life. Thus, it was realized that the pharmacological treatment of psychiatric disorders associated with fibromyalgia improves the condition of the patient's acceptance of the disease. Most medications had a good impact on the patient's quality of life without major side effects. It is known that adverse events are proportional to the dose of psychotropics, so for each patient, it is necessary to individualize the conduct
CONCLUSION: Antidepressants were the best-tolerated drug class, but antipsychotics, anticonvulsants, and other more recent drugs such as agomelatine were part of the study of the main drugs used in clinical practice
Keywords: fibromyalgia, psychotropics, treatment outcome.
Authors summary
Why was this study done?
The association between fibromyalgia and psychiatric disorders has been increasingly studied, mainly due to the favorable therapeutic advances achieved with the use of psychotropic drugs. Thus, the study can help professionals working in this area to take assertive behaviors regarding the treatment of fibromyalgia and expand scientific research on this topic, indirectly improving the clinical treatment of patients with this pathology.
What did the researchers do and find?
This is a systematic review study conducted in accordance with the guidelines of the Preferred Reporting Items for Systematic (PRISMA). We analyzed 23 articles, and the medications used in the studies were: Acetyl-L-carnitine, duloxetine, quetiapine, milnacipran, pregabalin, amissulpride, venlafaxine, mianserin (Lerivon®), mirtazapine, agomelatine. Increasingly, doctors and patients realize the need for multidisciplinary care for this clinical condition. In addition to antidepressants, it was seen that antipsychotics and anticonvulsants could also be used by patients to improve quality of life when used alone or in combination with serotonin or norepinephrine reuptake inhibitors.
What do these findings mean?
It has been seen that for successful treatment, antidepressants are well used, such as duloxetine and milnacipran, which comes to break with the outdated ideology that the gold standard medication in the treatment of fibromyalgia is amitriptyline. This study is an update for medical professionals of different specialties, mainly rheumatologists and psychiatrists so that they can better guide patients' morbidity as they use these medications to manage the patient with fibromyalgia.
INTRODUCTION
Fibromyalgia (FM) is a chronic medical condition characterized by generalized musculoskeletal pain that persists for at least 3 months, along with the presence of at least 11 of the 18 tender points on the exam. In addition, many patients also experience fatigue, mood disorders, headache, sleep disorders, and cognitive impairment1.
This rheumatological condition is often debilitating because it has important symptoms of pain that are characterized by myalgia and muscle sensitivity and may be accompanied by asthenia, stiffness, anxiety, sleep disorders, and depression. The clinical condition is quite common and occurs in about 2% of the general population. The pathophysiology of fibromyalgia is still unknown; however central monoaminergic neurotransmission plays an important role in its etiology2.
In states of pathological pain, pain inhibitory mechanisms may be dysfunctional, contributing to central sensitization and spinal and supraspinal hyperexcitability, generating uninterrupted transmission of neuronal pathways, and manifesting as persistent pain3.
The association between fibromyalgia and psychiatric disorders has been increasingly studied. In recent years, the psychiatrist has become more in demand with regard to his treatment, mainly due to the therapeutic advances achieved with psychotropic drugs, having, therefore, a more favorable treatment outcome⁴.
Thus, the objective of this study is to verify the response, tolerability, and adverse events of the use of psychotropic drugs in the treatment of fibromyalgia.
METHODS
A systematic review of articles on the use of psychotropics in fibromyalgia published in the chosen electronic databases was carried out. A literature search was conducted online using the MEDLINE database (via PUBMED) in September 2020, with no time limitation. Initially, the terms searched in the MEDLINE database were:
a) #1 "fibromyalgia" (MeSH Terms);
b) #2 "psychotropic drugs" (MeSH Terms);
c) #3 "treatment outcome" (MeSH Terms).
Descriptors were written in quotation marks. The following searches were carried out #1 AND #2 AND #3.
The research and the articles captured were reviewed three times on three occasions to ensure an adequate sample. The analysis of the articles took place after determining their relevance for the study. The PRISMA protocol5 was used for systematic literature review. The following inclusion criteria were obeyed: a) article whose titles refer to the topic addressed; b) studies dealing with psychotropic medications and fibromyalgia; c) original online articles accessible to the full text; d) prospective (cohort) or retrospective (case-control), observational (analytical or descriptive, except for case reports), experimental randomized clinical trials) or quase-experimental studies (open trials). Exclusion criteria were: a) other projects, such as case studies, case series, literature review, and comments; b) non-original studies, including editorials, reviews, prefaces, and letters to the editor. The work methodology was described in figure 1.
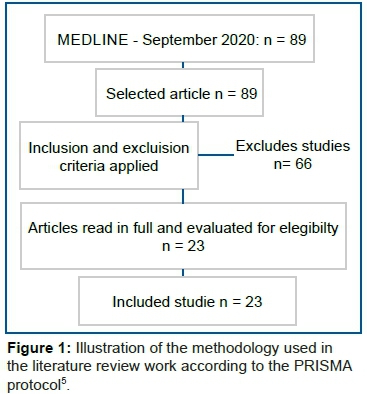
Then each study was read in its entirety, and the data were extracted and placed in table 1 that includes authors, year of publication, description of the study, sample, main study findings. Some of the studies did not deal exclusively with fibromyalgia but also with psychiatric disorders such as depression.
RESULTS
DISCUSSION
Of the 23 selected articles, 13 are experimental studies (randomized clinical trials), three are prospective studies (cohort), a retrospective study (case-control), and six quase-experimental studies (open trials). Of the randomized clinical trials, five used only female individuals as a sample2,6-9. Eight used a sample of both sexes1,10-16. However, the prevalence of the sample was higher for females. It was noticed that there is no uniformity between clinical trials when it comes to the age group of the sample. Most of the randomized clinical studies did not specify the race of the patients; however, four articles cited that almost all of the sample was of Caucasian individuals1,2,8,10.
The psychotropic medications used in these studies were: Acetyl-L-carnitine, duloxetine, quetiapine, milnacipran, pregabalin, amitriptyline, nortriptyline. Only one article cited acetyl-L-carnitine as a promising medication in the treatment of FM6, while 6 articles tested duloxetine as an effective drug in FM1,2,6,11,12,15. Two studied quetiapine8,14. Four tested the effectiveness of milnacipran7,9,10,13. Only 1 cited pregabalin as the medication used in the sample12, and one used amitriptyline and nortriptyline16.
Regarding longitudinal studies, only two articles do not specify gender17,18. Two estimated mean age of 44 years and 47.7 (standard deviation ± 8.6 years)19,20. None of these four studies made reference to a specific breed. The medications used were amitriptyline, duloxetine, gabapentin, and pregabalin17. Pregabalin in combination with trazodone20, olanzapine19, and tetracyclic antidepressant mianserin (Lerivon®)18.
Among the open studies, five of them refer to both sexes when choosing the sample2,21-24. Four studies specified the age group of people over 18 years of age4,22-24. The race of the sample was not mentioned in any of the four articles, and the medications studied were quite different: quetiapine XR and amitriptyline22, mirtazapine3, agomelatin4, trazodone and pregabalin21, venlafaxine23, amilsuprida24.
Randomized clinical trials
Female or both sexes taking Duloxetine
Leombruni et al.6 used Acetyl-L-carnitine and duloxetine, demonstrating the therapeutic efficacy of both medications in controlling FM symptoms. Arnold et al.2 found that the use of duloxetine at a dose of 60mg in 12 weeks had a positive and independent impact on the intensity of pain and mood compared to placebo. Moore et al.15 demonstrated that the response rates with the use of duloxetine reached 28% in the second week after the beginning of the use when compared to the placebo, which reached a reduction of pain in the order of 18% after 6 weeks of use.
García-Campayo et al.12 stated that pharmacological interventions and psychotherapeutic treatments for fibromyalgia were effective, and catastrophization was considered one of the most important modulating variables in the experience of pain.
Mease et al.1 used the same dose of duloxetine in the study by García-Campayo12, varying the dose from 60-120mg daily. Patients who used higher doses of the antidepressant had a higher dropout rate due to side effects, such as blurred vision, urinary retention, sedation, and weight gain. There was a sustained improvement in the average pain score in patients who remained on the 60mg dose a day or who increased the dose. Arnold et al.11 found that treatment with duloxetine significantly improved the multiple dimensions of fatigue, including pain, anxiety, depressed mood, stiffness, and sleep difficulties.
Female or both sexes using Quetiapine
McIntyre et al.14 showed that quetiapine was well tolerated at an average dose of 224mg per day, despite causing changes in the lipid profile (elevated triglycerides and reduced HDL) and weight gain when compared to placebo. However, these changes are consistent with the expected metabolic profile of the drug.
Potvin et al.8 also showed that quetiapine was effective on sleep disorders, revealing significant improvements in the items "rested", "anxious," and "depressive". However, no benefits were found on the physical symptoms of FM. The adverse effects found in the patients were elevated triglycerides, decreased HDL cholesterol, drowsiness, dry mouth, among others, some of them congruent with the findings in the study by McIntyre et al.14. In order for quetiapine to have an analgesic effect on the physical symptoms of FM, thus altering the outcome of tender points, the authors suggest that the dose should be high, varying from 150 to 300mg/day8.
Female or both sexes using Milnacipran
Milnacipran, a non-selective antidepressant for the reception of serotonin and norepinephrine, was used by Matthey et al.7 to treat the painful component of FM. It was observed that the medication reduces the pain and improves the quality of life in FM regardless of the emotional state of the patients. Arnold et al.10 showed that patients who received milnacipran (MNL) at a dose of 100mg per day had an improvement in mood and pain. In the study by Gendreau et al.13, the drug was also well-tolerated, with no serious adverse events. The study by Jensen et al.9 also claimed that MNL is correlated with the reduction of clinical pain.
Both sexes using Amitriptyline and Nortriptyline
Heymann, Helfebstein, and Feldman16 observed that patients who used 25 mg in the amitriptyline, nortriptyline, and placebo groups in 8 weeks had an improvement in FM symptoms. Improvement was seen in 36.5% in the amitriptyline group, 26.7% in the nortriptyline group, and 24% in the placebo group.
Longitudinal Studies
Kim, Landon, and Solomon17 showed that depression and anxiety were significant factors in initiating duloxetine compared to gabapentin. Abdominal pain, sleep disturbance, and inflammatory arthritis significantly increased the patient's chance of being treated with pregabalin compared to gabapentin. These findings suggest that the majority of patients with FM and taking one of the four prescribed medications were using inadequate doses, thus demonstrating the need to improve the overall management of fibromyalgia with respect to patient education, titration of adequate dose for pharmacological treatments, and non-pharmacological management strategies, such as aerobic exercise.
Rivera et al.20 found that the administration of an antidepressant or an anticonvulsant improves the patient's symptoms. However, when the two classes of medications are added at the same time (pregabalin and trazodone), the effect on improving FM symptoms increases by 50% when compared to the isolated use of the antidepressant and by up to 100% when compared to the isolated use of the anticonvulsant.
The retrospective study by Freedenfeld et al.19 found that olanzapine was effective in improving FM symptoms in patients who had limited success with other treatment modalities.
Tabeeva et al.18 showed a clinical effect in decreasing the intensity of the pain syndrome and autonomic manifestations, as well as improving nighttime sleep in the group that used the antidepressant mianserin (Lerivon®). The administration of mianserin (Lerivon®) or ibuprofen (Nurofen®) promoted an increase in pain thresholds (according to the flexor nociceptive reflex data).
Open Essay
Calandre et al.22 compared the efficacy and tolerability of prolonged quetiapine release (Seroquel XR®) and amitriptyline in the treatment of fibromyalgia. It was found throughout the study that quetiapine XR is poorly tolerated and does not provide similar efficacy to amitriptyline in patients with FM.
Bruno et al.4 showed that agomelatine was well tolerated, being effective in reducing pain, sleep disturbance, daytime fatigue, and depression despite not having a significant impact on neuropsychological characteristics (executive/cognitive symptoms).
The open study by Calandre et al.22 showed that treatment with trazodone significantly improved the overall severity of fibromyalgia, depression, and the impact of pain on activities of daily living, without demonstrating a direct effect on painful symptoms. Pregabalin played an additional role in improving physical pain when combined with trazodone.
Samborski, Lezanska-Szpera, and Rybakowski3 demonstrated that mirtazapine at a dose of 30mg per day is effective in reducing the intensity of pain, sleep disorders, fatigue, intensity of vegetative and functional symptoms, being the most robust effect of the medication on the quality of the sleep.
Dwight et al.23 used venlafaxine at a dose of 150 to 300mg. Visual analog individual scales of pain, fatigue, quality of sleep, sensation on waking, and morning stiffness showed significant improvement. However, this study had a very small sample, limiting its results.
Rico-Villademoros et al.24 have shown that amissulpride does not appear to offer any benefit to patients with fibromyalgia. Amissulpride was poorly tolerated by study participants.
The medications used in the studies were: Acetyl-L-carnitine, duloxetine, quetiapine, milnacipran, pregabalin, amissulpride, venlafaxine, mianserin(leviron®), mirtazapine, agomelatine. Most of the selected articles had clear information about the sample, type, and duration of the study but did not have definitive conclusions about the treatment outcomes with the studied medications. A large part of the studies had a female and caucasian sample, possibly because it was the group of patients most affected by the condition.
Clinical perspective
What's new?
It has been seen that for successful treatment, antidepressants are well used, such as duloxetine and milnacipran, which comes to break with the outdated ideology that the gold standard medication in the treatment of fibromyalgia is amitriptyline. In addition to antidepressants, it was seen that antipsychotics and anticonvulsants can also be used by patients to improve quality of life when used alone or in combination with serotonin or norepinephrine reuptake inhibitors.
What are the clinical implications?
The improvement in the quality of life of the patients in this study was directly linked to lower levels of pain. Most of the adverse effects were not an impediment to the continuation of the studies and were directly related to the increase in the dose of medications. Thus, more and more, doctors and patients realize the need for multidisciplinary care for fibromyalgia. The knowledge of the psychotropics used in the treatment, including the efficacy of different classes and the adverse effects found after their administration, are important for the management of the pathology, considering that most of the patients did not have serious side effects and had good tolerability.
CONCLUSION
Antidepressants were the best-tolerated drug class, but antipsychotics, anticonvulsants, and other more recent drugs such as agomelatine were part of the study of the main drugs used in clinical practice, with satisfactory clinical response and low risks of adverse effects.
REFERENCES
1.Mease PJ, Russell IJ, Kajdasz DK, Wiltse CG, Detke MJ, Wohlreich MM, et al. Segurança a longo prazo, tolerabilidade e eficácia da duloxetina no tratamento da fibromialgia. Seminários em Artrite e Reumatismo. junho de 2010; 39(6): 454-64. [ Links ]
2.Arnold LM, Rosen A, Pritchett YL, D'Souza DN, Goldstein DJ, Iyengar S, et al. A randomized, double-blind, placebo-controlled trial of duloxetine in the treatment of women with fibromyalgia with or without major depressive disorder. Pain. dezembro de 2005; 119 (1-3): 5-15. [ Links ]
3.Samborski W, Lezańska-Szpera M, Rybakowski JK. Effects of antidepressant mirtazapine on fibromyalgia symptoms. Rocz Akad Med Bialymst. 2004; 49:265-9. [ Links ]
4.Bruno A, Micò U, Lorusso S, Cogliandro N, Pandolfo G, Caminiti M, et al. Agomelatine in the treatment of fibromyalgia: a 12-week, open-label, uncontrolled preliminary study. Journal of Clinical Psychopharmacology. agosto de 2013; 33 (4): 507-11. [ Links ]
5.Moher D, Liberati A, Tetzlaff J, Altman DG, The prisma Group. Principais itens para relatar revisões sistemáticas e meta-análises: A recomendação PRISMA. Epidemiol. Serv. Saúde. 2015; 24 (2): 335-342. [ Links ]
6.Leombruni P, Miniotti M, Colonna F, Sica C, Castelli L, Bruzzone M, et al. A randomised controlled trial comparing duloxetine and acetyl L-carnitine in fibromyalgic patients: preliminary data. Clin Exp Rheumatol. 2015; 33 (1 Suppl 88). [ Links ]
7.Matthey A, Cedraschi C, Piguet V, Besson M, Chabert J, Daali Y, et al. Dual reuptake inhibitor milnacipran and spinal pain pathways in fibromyalgia patients: a randomized, double-blind, placebo-controlled trial. Pain Physician. outubro de 2013; 16 (5): E553-562. [ Links ]
8.Potvin S, Morin M, Cloutier C, Gendron A, Bissonnette A, Marchand S. Add-on treatment of quetiapine for fibromyalgia: a pilot, randomized, double-blind, placebo-controlled 12-week trial. Journal of Clinical Psychopharmacology. outubro de 2012; 32 (5): 684-7. [ Links ]
9.Jensen KB, Petzke F, Carville S, Choy E, Fransson P, Gracely RH, et al. Segregating the cerebral mechanisms of antidepressants and placebo in fibromyalgia. The Journal of Pain. dezembro de 2014; 15 (12): 1328-37. [ Links ]
10.Arnold LM, Palmer RH, Gendreau RM, Chen W. Relationships among pain, depressed mood, and global status in fibromyalgia patients: post hoc analyses of a randomized, placebo-controlled trial of milnacipran. Psychosomatics. julho de 2012; 53 (4): 371-9. [ Links ]
11.Arnold LM, Wang F, Ahl J, Gaynor PJ, Wohlreich MM. Improvement in multiple dimensions of fatigue in patients with fibromyalgia treated with duloxetine: secondary analysis of a randomized, placebo-controlled trial. Arthritis Res Ther. 2011; 13 (3): R86. [ Links ]
12.García-Campayo J, Serrano-Blanco A, Rodero B, Magallón R, Alda M, Andrés E, et al. Effectiveness of the psychological and pharmacological treatment of catastrophization in patients with fibromyalgia: a randomized controlled trial. Trials. dezembro de 2009; 10 (1): 24. [ Links ]
13.Gendreau RM, Thorn MD, Gendreau JF, Kranzler JD, Ribeiro S, Gracely RH, et al. Efficacy of milnacipran in patients with fibromyalgia. J Rheumatol. outubro de 2005; 32 (10): 1975-85. [ Links ]
14.McIntyre A, Paisley D, Kouassi E, Gendron A. Quetiapine fumarate extended-release for the treatment of major depression with comorbid fibromyalgia syndrome: a double-blind, randomized, placebo-controlled study: quetiapine xr for patients with mdd and fibromyalgia. Arthritis & Rheumatology. fevereiro de 2014; 66 (2): 451-61. [ Links ]
15.Moore RA, Cai N, Skljarevski V, Tölle TR. Duloxetine use in chronic painful conditions - Individual patient data responder analysis. European Journal of Pain (United Kingdom) [Internet]. 2014 Jan [cited 2021 May 24]; 18(1): 67-75. Available from: https://pubmed.ncbi.nlm.nih.gov/23733529. [ Links ]
16.Heymann RE, Helfenstein M, Feldman D. A double-blind, randomized, controlled study of amitriptyline, nortriptyline and placebo in patients with fibromyalgia. An analysis of outcome measures. Cli Exp Rheumatol. 2001 Nov-Dec-PubMed [Internet]. [cited 2021 May 24]; 19(6):697-702. Available from: https://pubmed.ncbi.nlm.nih.gov/11791642. [ Links ]
17.Kim SC, Landon JE, Solomon DH. Clinical characteristics and medication uses among fibromyalgia patients newly prescribed amitriptyline, duloxetine, gabapentin, or pregabalin. Arthritis Care and Research [Internet]. 2013 Nov [cited 2021 May 24]; 65(11): 1813-9. Available: from:https://pubmed.ncbi.nlm.nih.gov/23861291. [ Links ]
18.Tabeeva GR, Levin IaI, Korotkova SB, Khanunov IG. Lechenie fibromialgii [Tratamento da fibromialgia]. Zh Nevrol Psikhiatr Im S S Korsakova. 1998 [cited 2021 May 24]; 98(4): 40-3.. Available from: https://pubmed.ncbi.nlm.nih.gov/9606898. [ Links ]
19.Rivera J, Vallejo MÁ, Esteve-Vives J, Alegre C, Belenguer R, Belmonte M, et al. Estrategias de prescripción de fármacos en el tratamiento de pacientes con fibromialgia. Reumatologia Clinica [Internet]. 2012 Jul [cited 2021 May 24];8(4):184-8. Available from: https://pubmed.ncbi.nlm.nih.gov/22609004. [ Links ]
20.Freedenfeld RN, Murray M, Fuchs PN, Kiser RS. Decreased pain and improved quality of life in fibromyalgia patients treated with olanzapine, an atypical neuroleptic. Pain Practice [Internet]. 2006 Jun [cited 2021 May 24]; 6(2): 112-8. Available from: https://pubmed.ncbi.nlm.nih.gov/17309719. [ Links ]
21.Calandre EP, Morillas-Arques P, Molina-Barea R, Rodriguez-Lopez CM, Rico-Villademoros F. Trazodone plus pregabalin combination in the treatment of fibromyalgia: A two-phase, 24-week, open-label uncontrolled study. BMC Musculoskeletal Disorders [Internet]. 2011 [cited 2021 May 24];12. Available from: https://pubmed.ncbi.nlm.nih.gov/21575194. [ Links ]
22.Calandre EP, Rico-Villademoros F, Galán J, Molina-Barea R, Vilchez JS, Rodriguez-Lopez CM, et al. Quetiapine extended-release (Seroquel-XR) versus amitriptyline monotherapy for treating patients with fibromyalgia: A 16-week, randomized, flexible-dose, open-label trial. Psychopharmacology [Internet]. 2014 [cited 2021 May 24]; 231(12): 2525-31. Available from: https://pubmed.ncbi.nlm.nih.gov/24398824. [ Links ]
23.Dwight MM, Arnold LM, O'Brien H, Metzger R, Morris-Park E, Keck PE. An open clinical trial of venlafaxine treatment of fibromyalgia. Psychosomatics [Internet]. 1998 [cited 2021 May 24];39(1):14-7. Available from: https://pubmed.ncbi.nlm.nih.gov/9538670. [ Links ]
24.Rico-Villademoros F, Rodriguez-Lopez CM, Morillas-Arques P, Vilchez JS, Hidalgo J, Calandre EP. Amisulpride in the treatment of fibromyalgia: An uncontrolled study. Clinical Rheumatology [Internet]. 2012 Sep [cited 2021 May 24]; 31(9): 1371-5. Available from: https://pubmed.ncbi.nlm.nih.gov/2. [ Links ]
 Correspondence:
Correspondence:
Juliane dos Anjos de Paula
julianepaula2@hotmail.com
Manuscript received: February 2021
Manuscript accepted: June 2021
Version of record online: July 2021














