Serviços Personalizados
Journal
artigo
Indicadores
Compartilhar
Revista de Etologia
versão impressa ISSN 1517-2805
Rev. etol. vol.10 no.1 São Paulo jun. 2011
ARTICLE
Aspects of the behavior and reproduction of Mastigoproctus brasilianus Koch, 1843, (Arachnida:Uropygi:Telyphonidae)
Aspectos do comportamento e reprodução de Mastigoproctus brasilianus (Koch, 1843)
Rodrigo Lopes FerreiraI; Wendell de Castro SilvaII; Vanessa Cristina VieiraII; Marconi Souza SilvaIII
I Universidade Federal de Lavras
II Escola Superior de Biologia e Meio Ambiente
III Centro Universitário de Lavras
RESUMO
A ordem Uropygi inclui aracnídeos sul tropicais predadores que apresentam pedipalpos raptoriais, um longo e multissegmentado pós-pigídio, e longo flagelo. O objetivo do presente trabalho foi avaliar o repertório comportamental de Mastigoproctus brasilianus (Koch 1843) em condições de laboratório, bem como descrever as diferentes formas de comportamentos envolvidos no ritual de acasalamento. Foram computados 25 atos comportamentais espontâneos. Os mesmos foram agrupados em 9 categorias que incluem: manter-se abrigado, permanecer imóvel, mover os apêndices sem deslocamento, extensão das pernas, limpar-se, mover-se com deslocamento (para frente ou para trás), cavar; se alimentar, "outras formas de comportamento" menos frequentes. As categorias de comportamentos diferiram entre os sexos e entre períodos de atividade. Os comportamentos dos machos diferem dos imaturos e das fêmeas adultas. O comportamento reprodutivo apresentado por M. brasilianus segue os padrões descritos para outras espécies.
Palavras-chave: Mastigoproctus. Uropygi. Neotrópicos. Comportamento.
ABSTRACT
The order Uropygi includes south tropical predator arachnids with large raptorial pedipalps, a long and multi-segmented pos-pygidium, and long flagellum. The aim of the present work is to evaluate the behavior repertoire of Mastigoproctus brasilianus (Koch 1843) in laboratory conditions, as well as describing the different forms of behavior involved in the mating ritual of this species. A total of 25 behavioral acts were witnessed, that were clustered in 9 categories which included: to remain sheltered; to remain immobile; to move appendices without displacement; extensioning the legs; to clean itself; to move (forward or backward); to dig; to feed and "other forms of behavior". The categories of behaviors varied between sexes and between periods of activity. Males differed from all the others and the females presented a similar pattern when compared to the immature forms. The reproductive behavior presented by M. brasilianus follows the standards described for other species.
Keywords: Brazil. Uropygi. Neotropics. Ethology.
Whip-scorpions (order Uropygi, Arachnida) includes south tropical predators with large raptorial pedipalps, a long and multi-segmented pos-pygidium, and a long flagellum. They also possess anal glands which are used with precision to spray a chemical cocktail containing acetic acid to dissuade the predator (Eisner et al., 1961) Reaching up to 10 cm in length, they are sexually dimorphic: males and females, when adults, are distinguishable by the size and form of pedipalp, which are usually small in females and large in males (Weygoldt, 1971). The Uropygi are restricted to tropical and semitropical regions, preferring moist sites.
The whip-scorpions reproduce by indirectly transferring sperm through a spermatophore. The male uses its pedipalps to press the spermatophore against the female's gonepore (Weygoldt, 1970, 1971). The copulating behavior includes a complex ritual that has already been documented for some species (Gravely, 1915; Kaestner, 1931; Weygoldt, 1970, 1988). From the egg laying to eclosion, the female remains in a shelter and maintains the eggs attached to a pouch on her body. The offspring, after ecloding, go through some moltings and disperse, and the female ends up dying after the offspring dispersion.
Most of the works on Uropygi are description of species (Rowland, 2002; Viquez & Armas, 2007). There is also some reference to the mating repertoire of some species, but just a few concerning non-mating behaviors presented by the organisms (Crawford & Cloudsley-Thompson, 1971; Weygoldt, 1971).
Since the knowledge concerning the non-mating behaviors of many species of whip-scorpions is still incipient, the aim of the present work is to provide a behavior repertoire of Mastigoproctus brasilianus (Koch, 1843) in laboratory conditions, as well as describing the different forms of behavior involved in the mating ritual of this species.
Methods
Species habitat
The individuals come from two distinct populations, the townships of São José da Safira and Novo Oriente de Minas, both located within the Brazilian Atlantic forest biome in the Eastern and Northeastern of the Minas Gerais state. The individuals sheltered mainly under rocks and under fallen trunks present.
Anthropic activities in forest fragments have been modifying microhabitats and maintaining the landscape fragmented. Amongst these activities are the small-scale mining and the removal of fallen trunks from the undergrowth for the manufacture of charcoal.
Capture, transport and maintenance of specimens in laboratory
Six individuals were collected in August, 2003 in the cities of São José da Safira and Novo Oriente de Minas (three in each township). Two males and a female were collected in Novo Oriente de Minas. A female and two nymphs were collected in São José da Safira. The individuals collected were conditioned in plastic pots and transported to the laboratory of Underground Ecology of ESMA (Escola Superior de Meio Ambiente – Iguatama, MG, Brazil). The laboratory consists of an aphotic room climatized with temperature varying between 22ºC and 28ºC. The moist was maintained high (around 80%), simulating the prevailing conditions of the original habitats of the species.
In laboratory, each individual was placed in a terrarium (50cm length x 35cm width x 30cm height), with a layer of sand of 8 cm thickness on the bottom. The substratum was humidified once a week. Wood fragments were placed on the substratum to provide shelter for the organisms (one in each terrarium). Finally, a small plastic pot with water was placed for eventual ingestion of liquid. Preys (crickets and cockroaches, of unidentified species) were offered every 15 days to each individual.
During the observations, a red light was used in order to minimize the effect of artificial illumination on the photosensitive animals.
Non-mating behaviour
One-hour daily observations were made during 6 months (from August, 2003 to February, 2004). These observations aimed to qualify the array of behaviors and witness the behavioral repertoire of the individuals.
After qualifying the behaviors, 144 hours of observation were made in order to quantify different forms of behavior. These observations were divided among 6 individuals, resulting in a total of 24 hours for each individual. Each individual was observed daily for a period of 2 hours, varying the time of the day until a 24-hour cycle was completed for each individual. The observations of each individual started in the interval between 6 a.m. and 8 a.m. in the first observation day and finished in the interval between 4 a.m. and 6 a.m., 12 days after the begining of the observations. So, different behaviors were quantified in the morning, afternoon and night, for each individual. A chronometer was used to quantify the time elapsed in each behavior for each individual.
The animal-focal method was used for the behavioral description of the organisms. The measures are expressed in the amount of time that the observed organism remained showing a certain behavior. The ad libitum method was also used (Altmann, 1984).
Reproductive behavior
Reproductive behavior was observed by placing males and females in the same terrarium. The males were introduced in the female's terrarium. Each male was placed with each female separately, totalizing four mating episodes per couple. The forms of behavior presented by the individuals during each coupling repertoire were described.
Data analyses
For the analyses, the different forms of behavior observed were clustered in 10 categories, which will be described bellow. Activity time was here defined as all observed movements of the individuals (with or without displacements).
Results
For Mastigoproctus brasilianus a total of 24 behavioral acts were witnessed (Table 1). Not all individuals presented all the 24 behavioral acts observed. These behavioral acts represent the sum of behaviors observed in the six individuals studied. The different behaviors were clustered in nine categories for analysis, as aforementioned (Table 1).
Table 1. Behavior repertory of Mastigoproctus brasilianus (Uropygi, Thelyphonidae) in captivity conditions..
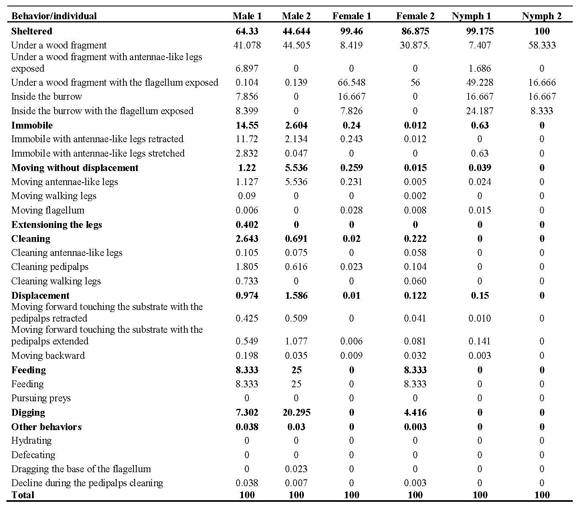
Hunting, defecating and hydrating behaviors were not observed during the quantification of behavior. These were only observed during the behavioral qualification phase.
Non-mating Behaviors
To remain sheltered was the most frequent behavior in individuals of this species in laboratory conditions (Table 1).
This category is subdivided in 5 different behavioral acts. The organisms sheltered themselves in burrows that they built or under wood fragments. When sheltered, the whip-scorpions' flagellum generally remained exposed. Although all the individuals preferentially remained sheltered, the males remained less time than the females and nymphs. The nymphs remained sheltered during almost all the behavioral quantification time (99.59% of the observations registered).
The organisms remained motionless at certain moments during behavioral quantification. This immobility was observed in the organisms exposed. The males and females remained motionless for more time than the nymphs due to the fact that they remained more time out of the shelters. When motionless, the organisms kept their flagellum straightened and in horizontal position, and the antennae-like legs extended.
When the whip-scorpions were not moving, they presented great activity in the movement of the antennae-like legs, continuously feeling the area in front of their body. These movements were performed by one of the legs at a time or both together. The males displayed this behavior more intensively than the females and the nymphs, having spent 3.38% of the time observed in this activity. The females spent 0.14%, and nymphs 0.02% of the time displaying this behavior.
During the periods of rest, only among the males, a behavior of "extensioning" (stretching) the locomotive appendices was observed. The individuals remained still and stretched each leg slowly, one by one. Each leg was extended and later bent, returning to the rest position. The organism alternated sides while stretching the legs, stretching a leg from the right side of the body and then one from left side of the body. This behavior would continue until all the legs had been stretched. Such behavior was rare, having been observed only in 0.2% of the observation time.
During the locomotive activity, the organisms slowly walked around the terrarium feeling the surrounding environment with their antennae-like legs. During this behavior, the flagellum was often straightened and parallel to the substratum. The males displayed this behavior more intensively than the females and nymphs, having spent 1.28% of the time observed in this activity, while females 0.065% and nymphs 0.075%.
The specimens frequently excavated the floor of the substratum in the terrarium to build their burrows. Using their pedipalps as shovels, they gathered the sand in mounts and transported them from their future burrow, depositing this material in disposal mounts, always located some centimeters from the entrance of the burrow. These organisms built and used several burrows, with different sizes and number of entrances. The construction of burrows with one, two and even three entrances was observed. Some burrows were used as living space while others were used only as shelters during feeding. In the latter, the organisms only remained inside the burrow while feeding on prey captured on the terrarium's ground, that were then carried to the feeding burrows. At the end of feeding, the organisms discarded the carcasses in its interior, thus also using the burrows as "dumpsters". The males presented excavating behavior more intensively than the females and nymphs, having spent 13.8% of the time observed in this activity. The females had only used 2.21% of the observation excavating burrows, and the nymphs were not observed building burrows during the quantification of behavior. The activity of the M. brasilianus in terrarium was realized manly along of the night and morning start, 18h00min until 06h00min (Figure 1).
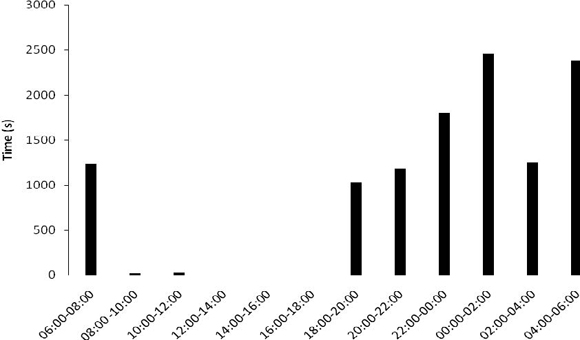
Figure 1. Activity time observed in M. brasilianus in a terrarium..
Feeding behavior
As soon as the potential prey (cricket or cockroach) approached the whip-scorpion it was quickly noticed. After approaching the prey, the whip-scorpion assumed an attack/defense position, remaining still for some seconds with the antennae-like legs and pedipalps opened, and the flagellum straightened in the upright position. After some seconds remaining still, the organism started to feel the prey with the antennae-like legs. This was done through fast touches in the prey. Such process occurred in such a delicate way, that the prey was not scared by the smooth touch of the whip-scorpion. The latter started the inspection by the frontal part of the prey. The antennae-like legs slowly felt the prey from the frontal part to its lateral portions, with soft touches on the flanks and on the dorsal portions of the prey. If the latter presented an unfavorable size the whip-scorpion would withdraw and hide. This behavior was witnessed 3 times. However, if the size of the prey was favorable for the attack by the whip-scorpion (in general of similar or inferior size to the Uropygi´s size), the latter would approach in a sudden and fast way, closing its pedipalps, in order to immobilize and squeeze the prey. After captured, the prey was torn by the chelicera and the Uropygi started to ingest small fragments. The feeding lasted in general several minutes. At the end, only a disformed mass of sclerified remains from the prey were left, being later discarded by the whip-scorpion.
This feeding behavior was more frequently observed among the males (16.67%) when compared with the females and the nymphs. The females and nymphs, although being fed, did not have a specific feeding behavior during the period of behaviors quantification (Table 1).
After feeding, the whip-scorpion cleaned its pedipalps, taking them up to the chelicera, rubbing them one against the other or using the second pair of legs to clean them more laterally. The antennae-form legs were also taken up to the chelicera to be cleaned. The cleaning of the flagellum, although less frequent, was also observed. In this case, the opistosoma was dorsally folded over the prosoma, thus allowing the flagellum to be caught by the chelicera. The cleaning started from the base of the flagellum to the extremity. The males presented cleaning behavior more intensively than the females, having spent 1.69% of the time observed in this activity, while females only 0.12%. Nymphs were not observed cleaning the pedipalps (Table 1).
When defecating, the organism raised the metasoma, promoting the opening of the anus and the release of excrements, that are oval and of dark brown coloration. In two occasions organisms were observed dragging the base of the flagellum on the sand. The metasoma was dragged on the substratum while the flagellum was kept straightened up. The organism would drag the flagellum, for some centimeters and later it would stop, remaining motionless. This rare behavior was only observed among the males. The behavioral act of moving backward (withdrawing) was relatively expressive when the organism left its burrow or when it found barriers ahead. Once more, the males revealed this behavior more frequently than the females, having spent 0.12% of the time observed in this activity, while females only 0.02% (Table 1).
Reproductive behavior
After placed in the same terrarium, the individuals could feel the presence of each other. This can be inferred by the fact that both quickly raised the flagellum when in the presence of each other. After some seconds, the male started to move slowly to the female. As soon as it came close to the female, it started to touch the latter with its antennae-like legs, maintaining the pedipalps opened as in the attack position. The female then placed herself in the attack/defense position, and started to move the abdomen rhythmically, raising and lowering it in fast movements (Figure 2A). Such behavior lasted about one minute and, during this time, the female gradually reduced the frequency of rhythmic movements, until she finally stopped. During this exhibition of the female, the male remained still, with only its antennae-like legs in contact with the female´s body. After this period the male felt the female with the antennae-like legs and both would keep touching each other until they were face-to-face, remaining still for approximately two minutes. When the female presented some resistance, the male would act with aggressiveness, and would drag the former with his pedipalps, positioning the female in front of him.
After the acceptance by the female, both would touch each other again and would keep the abdomen moderately raised. The male would then hold the female´s antennae-like legs with his palpal fingers and position them near his chelicera (Figure 2B). The male would first cross the female´s antennae-like legs before placing their extremities in his chelicera. Thus, the left female´s antennae-like leg joined the left male´s and vice versa (both facing each other) (Figure 2C). From this moment the male slowly manipulated the extremities of the female´s antennae-like legs with its chelicera. This behavior could last up to two hours (Figure 2D). After this period, the male slowly curved his body over the female´s body, without releasing her antennae-like legs, through a lateral turn (Figure 2E). During the process, the female´s antennae-like legs ended up being uncrossed, lining up with the corresponding male´s chelicera. The male was then positioned with the posterior region of his abdomen toward the female, so that her pedipalps would be attached to the male´s abdomen (Figure 2F). Both remained together and continued walking slowly and cautiously. The male and female remained attached to each other for a period of twenty-four to forty-eight hours. While they walked around the terrarium, the male apparently looked for sheltered places for the transference of spermatophore to the female. Unfortunately, such transference was not observed in any of the mating processes, since the organisms sheltered under pieces of fallen trunks (wood fragments) during this process. After release of the spermatophore by the male, they freed themselves (Figure 2G). In one of the unsuccessful mating, the spermatophore was observed adhered to the female´s lateral part of the body (Figure 2H).
Each couple was placed together in four distinct episodes, in which the mating behavior was observed. During the mating between male 1 and female 1, the release of 3 spermatophores occurred. During only one of the episodes the release of spermatophores did not occur. In the mating between male 1 and female 2 only one spermatophore was released. In the mating between male 2 and female 1, and male 2 and female 2 the release of spermatophores did not occur in any of the mating episodes.
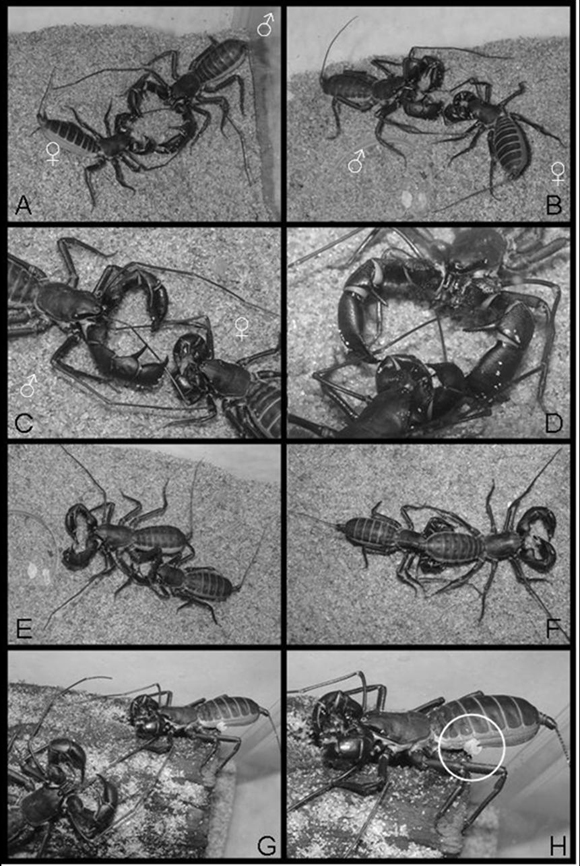
Figure 2. Reproductive behavior observed in M. brasilianus. See explanations in the text for details. Circle in H shows the spermatophore attached to the female´s abdomen.
Discussion
Although Weygoldt (1971) has made some studies on the life history of Mastigoproctus giganteus, in Brazil and in the rest of the world, studies concerning the biology of Uropygi are rare (Rowland & Adis, 2002). Thus comparing the forms of behavior presented by M. brasilianus with those observed in other species included in this order is more complicated.
The behavior of remaining sheltered can be frequent in the individuals in captivity and there seems to be a difference in this behavior related to the age and the sex of the individuals observed.
M. brasilianus has the capacity of digging its own burrows when in captivity. However, this is apparently rare in natural environments, where more than 10 organisms were observed to shelter themselves under fallen trunks or rocks. Víquez and Armas (2007) have observed that individuals of the Mastigoproctus ayalai species were found preferentially under fallen logs in tropical rain forest. Ferreira and Souza-Silva, (in prep.), while studying granitic caves of Northeastern Minas Gerais, found only one individual of M. brasilianus inside a burrow in a cave.
The water ingestion was only observed in a male during the qualification of behaviors. The whip-scorpions ingest water from moist substrata or during feeding (Ahearn, 1970; Crawford & Cloudsley-Thompson, 1971).
Since the nymphs and females spent more time sheltered, a higher locomotion activity was expected among the males. This higher activity presented by the males may be due to the necessity of inspecting large areas in search of females or even prey. The behavior involved in prey recognition (remain in an attack/defense position, verifying the prey with the antennae-like legs and with open pedipalps) can eventually be related to risk for the whip-scorpion, since the prey may be a potential predator.
The act of leaving the feeding remains inside the feeding burrows may indicate that M. brasilianus frequently builds new burrows, since the remains could promote the growth of fungi, some of which possibly pathogenic.
It is important to point out that some non-mating forms of behavior observed in M. brasilianus may be interdependent. It is possible to observe, for example, that the males clean themselves more frequently than the females and nymphs. They move themselves, excavate, manipulate prey and defecate more intensively than the females and nymphs. So, the cleaning behavior in males can eventually be related to their activity intensity.
The act of stretching the appendices may be related to muscular relaxation that may facilitate the activity of spontaneous locomotion or escape. However, such behavior was evidenced here in this species for the first time, and the elaboration and execution of further studies to allow a better understanding of this behavior is of utmost importance.
Amongst all the mentioned forms of behavior presented by whip-scorpions, the most studied is the reproductive behavior (Gravely, 1915; Kaestner, 1931; Weygoldt, 1971, 1972; Rowland & Adis, 2002). Weygoldt (1971) studied, in a very detailed way, the reproductive behavior of Mastigoproctus giganteus, having described all mating repertoire for this species.
In general, the reproductive behavior presented by M. brasilianus follows the standards described by Weygoldt (1971) for M. giganteus. The main differences are in how the males and females approach each other. In M. giganteus it occurs in a more aggressive way, while in M. brasilianus, in a more cautious and pacific way, rarely occurring any aggressiveness by the male (such as those behaviors that could cause injuries to the females). Another remarkable difference is related to the position where the female attaches the spermatophore to her body, after the male has deposited it on the substratum. M. giganteus females position themselves above the spermatophore, in a way that the spermatophore is attached next to the genital opercule. In M. brasilianus, however, the spermatophores were all observed next to the anterior laterals of the female opsistosoma. Rowland and Adis (2002) mentions that all this behavioral repertoire is generically applicable to species of Telyphonellus and Typopeltis, presenting more variations when compared to the behaviors presented by species of Thelyphonus (Weygoldt, 1988).
The behavior presented by the male, of manipulating the antennae-like legs crossed with the chelicera of the female in the first moments of mating, also occurs in other species, such as M. giganteus (Weygoldt, 1971) and Thelyphonus sepiaris (Gravely, 1915).
Unfortunately, sperm transference was not observed in M. brasilianus. According to Weygoldt (1971), after the female attaches the spermatophore next to the body, the male, with its palpal fingers, pushes the sperm into the female's gonepore, a fact not evidenced in the present study. Besides that, the non-production of spermatophores in some mating acts, in laboratory, indicates that male 2 is probably sexually immature. Finally, the non-conclusion of some mating episodes may also be related to the conditions of stress of the individuals, due to captivity.
The register of M. brasilianus and the level of anthropic threats imposed categorize the region of Novo Oriente de Minas as priority area for the conservation of biodiversity in the State of Minas Gerais, Brazil (Machado & Ferreira, 2005). Therefore, the relevance of this species, as important taxa for conservation, is clear. It is of great importance that it becomes, once more, the aim of further studies on the new aspects of its biology.
References
Adis, J. (2002). Amazonian Arachnida and Myriapoda. Bulgaria: Pensoft Publishers. [ Links ]
Ahearn, G.A. (1970). Water balance in the whip scorpion, Mastigoproctus giganteus (Lucas) (Arachnida, Uropygi). Compared Biochemistry Physiology, 35, 339-353. [ Links ]
Altmann, J. (1984). Observational sampling methods for insect behavioral ecology. Florida Entomologist, 67(1), 50-56. [ Links ]
Crawford, C.S., & Cloudsley-Thompson. (1971). Water relations and desiccation-avoiding behaviour in the vinegaroon Mastigoproctus giganteus (Arachnida: Uropygi). Entomological Experimental Applied, 14, 99-106. [ Links ]
Eisner, T., Meinwald, J., Monro, A., & R. Gent. (1961). Defense mechanisms of Arthropods 1. The composition and function of the spray of the whipscorpion, Mastigoproctus giganteus (Lucas) (Arachnida:Pedipalpida). Journal of Insect Physiology, 6, 272-298. [ Links ]
Gravely, F.H. (1915). Notes on the habits of Indian insects, myriapods and arachnids. Records of the Indian Museum, 11, 483-539. [ Links ]
Kaestner, A. (1931). Ordnung der Arachnida-Pedipalpi Latreille Geisselskorpione. In: W. Kukenthal (Ed.). Handbuch der Zoologie (pp. 1-76), Berlin. [ Links ]
Machado, S.F., & Ferreira, R.L. (2005). Invertebrados. Biodiversidade em Minas Gerais: um Atlas para a sua conservação. Belo Horizonte: Fundação Biodiversitas. [ Links ]
Rowland, J. M. (2002). Review of the South American whip scorpions (Thelyphonida: Arachnida). Amazoniana, 17(1/2), 187-204. [ Links ]
Rowland, J.M., & Adis, J. (2002). Uropygi (Thelyphonida). In: J. Adis (Ed.), Amazonian Arachnida and Myriapoda. Bulgaria: Pensoft Publishers. [ Links ]
Víquez, C., & Armas, L.F. (2007). A new species of Mastigoproctus Pocock, 1894 (Thelyphonida: Thelyphonidae) from Venezuela. Zootaxa, 1463, 39–45.
Weygoldt, P. (1970). Courtship behavior and sperm transfer in the giant whipscorpion, Mastigoproctus giganteus (Lucas)(Uropygi, Thelyphonidae). Behaviour, 36, 1-8. [ Links ]
Weygoldt, P. (1971). Notes on the life history and reproductive biology of the Giant whip scorpion, Mastigoproctus giganteus (Uropygi, Theliphonidae) from Florida. Journal of Zoology, 164, 137-147. [ Links ]
Weygoldt, P. (1988). Sperm transfer and spermatophore morphology in the whipscorpion Thelyphonus linganus (Arachnida: Uropigyda: Thelyphonidae). Journal of Zoology, 215, 189-196. [ Links ]
Received October 21, 2010
Accepted 11, January, 2012
Rodrigo Lopes Ferreira
Laboratório de Ecologia Subterrânea
Setor de Zoologia
Departamento de Biologia
Universidade Federal de Lavras
Lavras, Minas Gerais, Brazil.
e-mail: drops@dbi.ufla.br