Serviços Personalizados
Journal
artigo
Indicadores
Compartilhar
Journal of Human Growth and Development
versão impressa ISSN 0104-1282versão On-line ISSN 2175-3598
J. Hum. Growth Dev. vol.29 no.2 São Paulo maio/ago. 2019
https://doi.org/10.7322/jhgd.v29.9428
ORIGINAL ARTICLE
Efficacy Evaluation of a non-human experimental model for ultrasound-guided superficial venous puncture: clinical randomized assay
Érica Patricio Nardino, *; Andrea Paula Kafejian-Haddad; Danilo Argollo Pirutti Silva; João Antonio Correa
Discipline of Angiology and Vascular Surgery, Faculdade de Medicina do ABC, Santo André, São Paulo, Brazil
ABSTRACT
INTRODUCTION: The use of a venipuncture simulator facilitates technique learning and improves skills, which reduces the risk of venipuncture complications in humans.
OBJECTIVE: To evaluate the efficacy of a non-human experimental model for Ultrasound guided superficial venipuncture.
METHODS: We randomized 39 nurses in two groups: A and B. The training had three steps: 1 - theoretical class, 2 - practical class, with the ultrasound device and 3 - ultrasound-guided puncture training in the non-human model. The group A participated in steps 1, 2 and 3 and group B in steps 1 and 2. After training, both groups were released for ultrasound guided venipuncture.
RESULTS: The success in puncture in group A (n = 20) was 90% and in group B (n = 19) it was 68.42%. In the sum of the identification and the puncture times, the average of group A was 61.5 seconds (95% CI, 33.58; 106.95) and in group B was 148.0 seconds (95% CI, 114.54; 208.44), which was statistically significant (p = 0.007, without overlapping the interval estimates.
CONCLUSION: Nurses who received training with the non-human model had better identification and puncture times.
Keywords: experimental model, ultrasound, venous puncture, clinical randomized essay
Authors summary
Why was this study done?
Training nurses to perform ultrasound guided venous puncture is an important issue to improve care for patients with indication for peripheral venous access, especially for those with difficult access.
What did the researchers do and find?
Researchers conducted a clinical randomized study to validate the effectiveness of a non-human model in the training of ultrasound guided venipuncture. A total of 39 nurses were randomized in two groups A and B to participate in a training that had 3 distinct stages. Nurses of group A participated in stages 1, 2 and 3 and those of group B (control group) participated in stages 1 and 2 of the training.
After training, nurses performed ultrasound guided venipuncture on patients, the variables were noted, among them, the identification and puncture times of the vein. It was observed that nurses who received stage 3 of the training were able to identify and puncture the vein in a shorter time, demonstrating the ability of the model used in step 3 to improve nurses' ability for the ultrasound guided venipuncture procedure.
What do these findings mean?
The validation of this low cost model and the methodology used in the training allow the qualification of professionals for ultrasound guided superficial venipuncture, improving the quality of care to patients, especially those with difficult access.
INTRODUCTION
The use of simulators makes it possible to advance in health education1, allowing the practice of procedures, improving the performance of health professionals in skills that require hand-eye coordination, such as the training for ultrasound-guided peripheral venous access (UGPVA), a clinical practice that is growing, since it has been reducing errors and increasing patient safety2-4.
The traditional superficial venous puncture depends on the location of the vein close enough to the skin to be visible or at least palpable, thus peripheral venous access (PVA), without the use of ultrasound, can be challenging, with a failure rate of approximately 25%5.
Ultrasound allows the identification of impalpable veins , its patency tested and its puncture performed under direct vision, which improves the success rates of PVA and decrease the complications3,4,6-8.
Up to 70% of patients require PAV during hospitalization9, so the delay in placing them, in the difficult cases, may cause harms to the patient due to increased discomfort, delayed diagnosis, in the beginning of the treatment, and may also lead the patient to a central venous access (CVA) which is more invasive, time-consuming and prone to severe complications such as pneumothorax, hemorrhage, infection, thrombosis, catheter displacement and air embolism4,8,10-13.
Thus, UGPVA has become an useful tool in cases of difficult puncture (when palpation and anatomical references fail) in noncritical patients14 and is an effective and safe alternative to CVA, which traditionally it is the next step after PVA failure15,16.
According to Oliveira and Lawrence 8, in recent years, the literature has shown that nurses have a high success rate in UGPVA placement.
Thus, the objective of this study is to analyze the effectiveness of a non-human experimental model in training nurses for ultrasound-guided superficial venipuncture.
METHODS
This randomized clinical trial was approved by the Ethics Committee of the Faculty of Medicine of ABC - CAAE Number 58123216.6.0000.0082, in October 19, 2016 with the number RBR - 9prvnm, in the brazilian registry of clinical trials. It was carried out at the Hospital Universitario Padre Anchieta (HUPA) in São Bernardo do Campo, Sao Paulo State, Brazil, between October 20, 2016 and April 1, 2017.
Os critérios de inclusão foram: fazer parte da equipe clínica da HUPA, concordar em participar do estudo e assinar o Termo de Consentimento Livre e Esclarecido ( Anexo S1) e não ter experiência com ultrassonografia ou punção guiada por ultrassonografia.
Among 55 nurses from the HUPA, 39 accepted to participate in this study, all female, from different hospital wards. The inclusion criteria were: be part of the hospital clinical staff; have no previous experience with ultrasound or ultrasound guided puncture and agree to participate in the study and signed a Free informed term of Consent ( FITC) (S1 Appendix).
The patients who participated in the study were hospitalized in different hospital wards with varied diagnoses and filled the following inclusion criteria: be admitted to the HUPA; have medical indication of peripheral venous access; be over 18 years old; present superficial vein in the upper limbs, with greater caliber than 0.35cm and up to 1.5cm deep (after ultrasound evaluation previously performed by the author of this work), agree to participate in the study and sign FITC (S2 Appendix);
Study stages
Nurses were randomly assigned to two groups A (n=20) and B ( n=19). Randomization was performed by the author of this study by lot. Each nurse took an envelope from a box containing the letters "A" or "B" to define which group would be part of.
Then, the groups A and B were subdivided into smaller groups of five to seven individuals. Nurses belonging to group A participated in steps 1, 2 and 3 and those of group B (control group) participated in the steps1 and 2.
The training was conducted in three stages, as follows:
Step 1: An hour of theoretical lecture, on the ultrasound device and the forms of identification of vascular structures (vein and artery), using ultrasound.
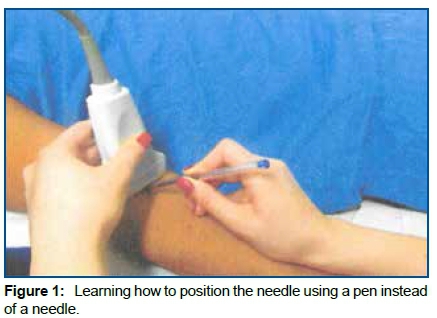
Step 2: During an hour of practical training on the Sonosite Titan ultrasound with 11MHz high frequency linear transducer. Nurses learned how to manipulate the device, position the transducer, identify the vein and test its patency in human volunteers. Position the needle with optimum angulation to the skin for venipuncture. At the time of the training, it was used a pen instead of a needle (Fig 1). Measure the diameter of the vein and its depth (distance from the center of the vein to the skin) to choose a catheter of adequate length. The puncture technique taught was the transverse axis with single operator.
The use of simulators makes it possible to advance in health education [1], allowing the practice of procedures, improving the performance of health professionals in skills that require hand-eye coordination, such as the training for ultrasound-guided peripheral venous access (UGPVA), a clinical practice that is growing, since it has been reducing errors and increasing patient safety [2-4].
The traditional superficial venous puncture depends on the location of the vein close enough to the skin to be visible or at least palpable, thus peripheral venous access (PVA), without the use of ultrasound, can be challenging, with a failure rate of approximately 25% [5].
Ultrasound allows the identification of impalpable veins , its patency tested and its puncture performed under direct vision, which improves the success rates of PVA and decrease the complications [3,4,6-8].
Up to 70% of patients require PAV during hospitalization [9], so the delay in placing them, in the difficult cases, may cause harms to the patient due to increased discomfort, delayed diagnosis, in the beginning of the treatment, and may also lead the patient to a central venous access (CVA) which is more invasive, time-consuming and prone to severe complications such as pneumothorax, hemorrhage, infection, thrombosis, catheter displacement and air embolism [4,8,10-13].
Thus, UGPVA has become an useful tool in cases of difficult puncture (when palpation and anatomical references fail) in noncritical patients [14] and is an effective and safe alternative to CVA, which traditionally it is the next step after PVA failure [15,16].
According to Oliveira and Lawrence [8], in recent years, the literature has shown that nurses have a high success rate in UGPVA placement.
Thus, the objective of this study is to analyze the effectiveness of a non-human experimental model in training nurses for ultrasound-guided superficial venipuncture.
METHODS
This randomized clinical trial was approved by the Ethics Committee of the Faculty of Medicine of ABC - CAAE Number 58123216.6.0000.0082, in October 19, 2016 with the number RBR - 9prvnm, in the brazilian registry of clinical trials. It was carried out at the Hospital Universitario Padre Anchieta (HUPA) in São Bernardo do Campo, Sao Paulo State, Brazil, between October 20, 2016 and April 1, 2017.
Os critérios de inclusão foram: fazer parte da equipe clínica da HUPA, concordar em participar do estudo e assinar o Termo de Consentimento Livre e Esclarecido ( Anexo S1) e não ter experiência com ultrassonografia ou punção guiada por ultrassonografia.
Among 55 nurses from the HUPA, 39 accepted to participate in this study, all female, from different hospital wards. The inclusion criteria were: be part of the hospital clinical staff; have no previous experience with ultrasound or ultrasound guided puncture and agree to participate in the study and signed a Free informed term of Consent ( FITC) (S1 Appendix).
The patients who participated in the study were hospitalized in different hospital wards with varied diagnoses and filled the following inclusion criteria: be admitted to the HUPA; have medical indication of peripheral venous access; be over 18 years old; present superficial vein in the upper limbs, with greater caliber than 0.35cm and up to 1.5cm deep (after ultrasound evaluation previously performed by the author of this work), agree to participate in the study and sign FITC (S2 Appendix);
Study stages
Nurses were randomly assigned to two groups A (n=20) and B ( n=19). Randomization was performed by the author of this study by lot. Each nurse took an envelope from a box containing the letters "A" or "B" to define which group would be part of.
Then, the groups A and B were subdivided into smaller groups of five to seven individuals. Nurses belonging to group A participated in steps 1, 2 and 3 and those of group B (control group) participated in the steps1 and 2.
The training was conducted in three stages, as follows:
Step 1: An hour of theoretical lecture, on the ultrasound device and the forms of identification of vascular structures (vein and artery), using ultrasound.
Step 2: During an hour of practical training on the Sonosite Titan ultrasound with 11MHz high frequency linear transducer. Nurses learned how to manipulate the device, position the transducer, identify the vein and test its patency in human volunteers. Position the needle with optimum angulation to the skin for venipuncture. At the time of the training, it was used a pen instead of a needle (Fig 1). Measure the diameter of the vein and its depth (distance from the center of the vein to the skin) to choose a catheter of adequate length. The puncture technique taught was the transverse axis with single operator.
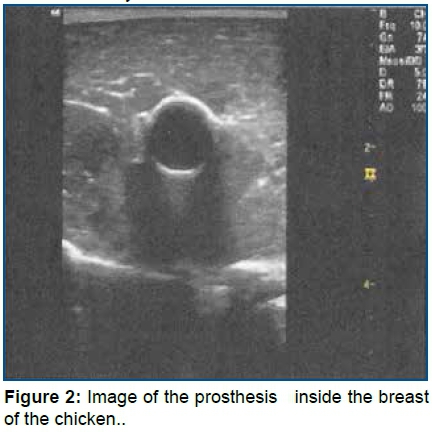
Step 3: Practice during an hour on Puncture training in the non-human training model made with chicken breast17. It was taught to identify the prosthesis inside of the non-human model that simulates a vein (Fig 2), to train the positioning of the transducer, the correct angulation of the needle in relation to the skin to perform guided puncture, identify the needle within the tissue and within the prosthetic structure that simulates a vein. Each nurse was able to perform the puncture as many times they found it necessary.
Soon after the training, nurses from both groups, A and B, were released for puncture in the participating patients, always supervised by the researcher of this study who collected the next variables:
- time for identification of the superficial superior limb vein by ultrasound, measured in seconds;
- time for puncture of the superficial superior limb vein, (including success cases and cases without success) measured in seconds;
- success (considered in the presence of reflux of blood);
- failure (considered after three attempts at puncturing, punctured the skin three times, no blood reflux);
- presence of minor complications (small bruises);
- presence of major complications (large bruises and arterial puncture).
The collection of these variables followed the steps below:
1. A nurse positioned the transducer on the skin and the timer was triggered. As soon as the vein was identified the timer was stopped and the time of identification of the vein was noted.
2. Local asepsis measurements were performed (with chlorhexidine alcohol 0.5%).
3. The nurse placed the transducer back into the skin, and when the needle was positioned on the skin, the timer was triggered. The puncture time was defined: a) Once the vein was successfully punctured (with the visibility of blood reflux by the research of this study) the timer was stopped and the puncture time was noted. b) As soon as the needle was pulled out of the skin after the third attempt (without the visibility of blood reflux by the scientist ) the timer was stopped and the puncture time was noted.
4. The presence of complication was noted.
5. An intravenous Jelco 20 catheter was used with 3cm in length Becton Dickinson BD® and the same ultrasound device used for training.
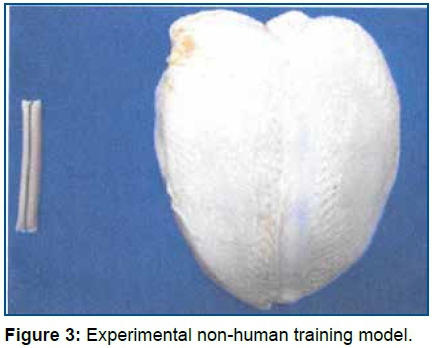
The non-human experimental training model17 (Fig 3) used in this work was developed with the use of chicken breast with skin, 6mm Dacron® prosthesis expired or discarded, sewing thread and needle and gelatin used in plant ornamentation. On the ultrasound, animage of a tubular structure was obtained, of anechoic and homogeneous content inside of the chicken breast musculature with ultrasound density similar to that of human muscle tissue. The model can be punched several times, since the gelatin does not leak from the prosthesis. Its current final cost was approximately R$ 8.00 per unit (around $2,50).
Statistical analysis
To determine the sample size, two sample averages were compared18,19, considering the following vein identification parameters: group A mean 33.57 and standard deviation 44.09 and group B mean 96.47 and standard deviation of 112.74. The confidence level adopted was 95%; sampling error of five percentage points, 80% test power.
Qualitative variables were described by absolute and relative frequencies. The quantitative variables were described by medians and 25% and 75% percentiles because they did not show adherence to normal distribution (Shapiro-wilk, P<0.05) The proportion of puncture successes and complications between the groups was analyzed through the Chi-square test with correction of Yates. For analysis of differences in puncture time and time of identification of the vein between the groups, it was used a Mann-Whitney test and interval estimates of medians. The level of significance was 5%. The program used was Stata11.0.
RESULTS
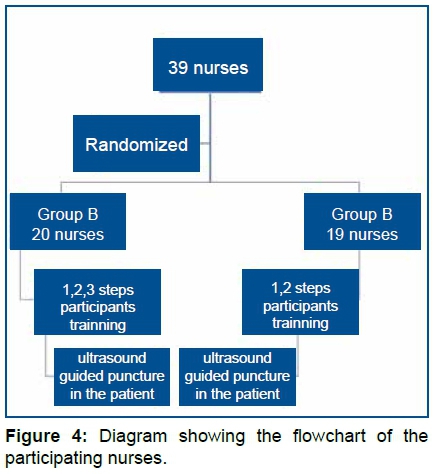
The figure 4 illustrate The nurses participating in the study (n = 39) were divided into groups A (n=20) and B (n=19) (Fig 4).
When analyzing the variable time of vein identification, the median time in group A was 18 seconds (95% CI, 5.23; 35.53) and, 48 seconds in the group B (95% CI, 21.16; 107.43), which was statistically significant, with p = 0.020, however, the intervals of confidence overlap themselves as well as the time of vein puncture, where the median time in group A was 34 seconds (95% CI, 18.46; 59.53) and 90 seconds in the group B(95% CI, 52.74; 120.61), which was statistically significant, with p = 0.029 but the intervals of confidence overlap themselves.
As shown in figure 5, 15 individuals from group A presented a time of identification of the vein shorter than 50 seconds and 5 individuals had a time longer than 50 seconds, being only 1 of them with time longer or equal to 150 seconds. In group B it is observed that 10 individuals had a vein identification time shorter than 50 seconds and 9 individuals had a time longer than 50 seconds, and 4 of them had time longer or equal to 150 seconds.
The figure 6 shows that 13 individuals of group A presented a time of puncture of the vein shorter than 50 seconds and 7 had a time longer than 50 seconds, and among these, only 1 presented longer or equal time to 150 seconds. In group B, 5 individuals had a time of puncture of the vein shorter than 50 seconds and 14 had a longer time than 50 seconds and, among these, 2 showed time longer or equal to 150 seconds.
Table 1 illustrates the sum of the time identification variables of the vein and time of vein puncture analyzed. The median of the sum in group A was 61.5 seconds (95% CI, 33.58; 106.95) and in group B, 148.0 seconds (95% CI, 114.54; 208.44), which was statistically significant (p = 0.007, without over lapping interval estimates)
From group A eighteen individuals had success in puncture and thirteen from group B , but this result was not statistically significant, with p = 0.095.
The percentage of veins punctured in order of frequency were, respectively, cephalic with 69.23%, intermediacy of the elbow with 17.95% and basis with 12.82%.
There was only one minor complication (small bruises) in the group B, but there was no statistically significant difference between the complications and the group with p = 0.299.
DISCUSSION
The success rate of ultrasound-guided puncture in group A was 90% (n = 18) and 68.42% in group B (n = 13), with an overall success of 79.5%. Although there was no statistically significant difference regarding success according to group, those who trained in the non-human model (group A) were more successful when compared to group B.
In the sum of the variables, identification time and vein puncture time, the median sum in group A was 61.5 seconds, and in group B 148 seconds, which was statistically significant (p = 0.007, without over lapping interval estimates), demonstrating the improvement in group skills that performed all 3 training stages.
When we evaluated the time, identification and puncture variables separately, both presented a shorter time in group A compared to group B, respectively 18 and 48 seconds and 34 and 90 seconds, which was statistically significant with p <0.05. (p = 0.020 and p = 0.029) but with overlapping confidence intervals.
However, when individually analyzing the identification time of each participant, it was noted that most of group A, 15 individuals , had a time under 50 seconds and only 1 of them with a time greater than or equal to 150 seconds. In group B, it was observed that 10 individuals (n = 19) had vein identification time less than 50 seconds and 4 had a time greater than or equal to 150 seconds, showing that most members of group A had shorter identification time.
The same observation can be made regarding venipuncture time, where 13 individuals from group A had venipuncture time less than 50 seconds and only 1 presented time greater than or equal to 150 seconds. In group B, 5 nurses had venipuncture time less than 50 seconds and 2 had a time greater than or equal to 150 seconds, showing that most members of group A had a shorter venipuncture time.
The shorter observed time of most individuals in group A, both in identification and puncture of the vein, reinforces the increase of their skills after the 3 stages of training.
When comparing the success rate of ultrasound-guided puncture in group A of 90%, and in the sum of groups (A and B) of 79.5%, we observed that the results are similar to those in the literature, which ranged from 63.2%. to 96.5%8, 20,21-23.
The summed or separated time values found are within the values described in the literature, ranging from 63.5 seconds to 28 minutes11,24,25,23,26.
In the literature, complication rates are low8,25,21, similar to those found in this study, with only a minor complication in group B.
To obtain the results discussed above, we chose to homogenize as much as possible the participants of this study, both nurses and patients. To avoid different levels of technical difficulty among nurses, and as the diameter and depth of the vein influence the success rate of ultrasound-guided puncture8,27-29, we chose to standardize a diameter (from 0.35 cm) and maximum depth (up to 1.5 cm) of the vein, as well as Meyer et al.30, instead of using patients with difficult access, since in the literature there is no standardization of criteria for their definition.
Among these criteria for difficult access are the number of puncture attempts (two or more)31,30,32, absence of visible and / or palpable vein in the upper limb32 and antecedent of the patient with difficult access10,31,33.
To prevent previously acquired skills from interfering with the results, only nurses with no previous experience in ultrasound or guided puncture participated in the study, as well as in Gopalasingam et al.20, unlike other studies in which participants were heterogeneous and with varied experiments8,33,34,24.
And finally, believing that the data collection by the nurses themselves could induce error, the author performed the data collection as well as Stein et al.35 and Mills et al.25.
These data suggest that the use of a low-cost, non-human model with a current value of R $ 8.00 (around $2,50) , similar to the equivalent values found in the literature ranging from US $ 3.00 to US $ 22.8334,36,37 or around € 24,0038, together with an easily reproducible training methodology, showed better technique results without complications, reinforcing that simulator training increases the rates of success by improving the technique and increasing the professional experience8,38 regardless of the financial conditions of the institutions.
In addition to the low cost, this model using chicken breast has a texture and echogenicity closer to human, unlike models made of gel or gelatin that can evolve with artifacts in the image (air injected into the gel along the rail made by the needle), have a very low echogenicity that exaggerates the visibility of the needle36 and may lead to false confidence regarding clinical capacity39.
This nursing staff training project is emerged from the need to benefit hospitalized patients with CVA indication after failure of PVA by traditional method, and that, after ultrasound evaluation by the medical team, non-visible and non-palpated veins were found and punctured, allowing maintenance of the PVA without the need for CVA.
CONCLUSION
The non-human experimental model used in the training of nurses for ultrasound-guided superficial venipuncture was able to increase their abilities, demonstrated by the shorter identification and venipuncture times in the group that trained in the non-human model (group A) in relation to the control group (group B). Despite a small sample, the results were statistically significant, showing that a reproducible training methodology and a low cost model allow adequate nursing staff training, improving the quality of patient care.
REFERENCES
1.Gorman PJ, Meier AH, Rawn C, Krummel TM. The future of medical education is no longer blood and guts, it is bits and bytes. Am J Surg. 2000;180(5):353-6. DOI: http://doi.org/10.1016/s0002-9610(00)00514-6 [ Links ]
2.Evans LV, Dodge KL, Shah TD, Kaplan LJ, Siegel MD, Moore CL, et al. Simulation training in central venous catheter insertion: improved performance in clinical practice. Acad Med. 2010;85(9):1462-9. DOI: http://doi.org/10.1097/ACM.0b013e3181eac9a3 [ Links ]
3.Di Domenico S, Santori G, Porcile E, Licausi M, Centanaro M, Valente U. Inexpensive homemade models for ultrasound-guided vein cannulation training. J Clin Anesth. 2007;19(7):491-6. DOI: http://doi.org/10.1016/j.jclinane.2007.05.002 [ Links ]
4.McGee DC, Gould MK. Preventing complications of central venous catheterization. N Engl J Med. 2003;348:1123-33. DOI: http://doi.org/10.1056/NEJMra011883 [ Links ]
5.Jacobson AF, Winslow EH. Variables influencing intravenous catheter insertion difficulty and failure: an analysis of 339 intravenous catheter insertions. Heart Lung. 2005;3495):345-59. DOI: http://doi.org/10.1016/j.hrtlng.2005.04.002 [ Links ]
6.Dietrich CF, Horn R, Morf S, Chiorean L, Dong Y, Cui XW, et al. US-guided peripheral vascular interventions, comments on the EFSUMB guidelines. Med Ultrason. 2016;18(2):231-9. DOI: http://doi.org/10.11152/mu.2013.2066.182.umb [ Links ]
7.Kuo CC, Wu CY, Feng IJ, Lee WJ. Efficacy of ultrasound-guided peripheral intravenous access: a systematic review and meta-analysis. Hu Li Za Zhi. 2016;63(6):89-101. DOI: http://doi.org/10.6224/JN.63.6.89 [ Links ]
8.Oliveira L, Lawrence M. Ultrasound-guided peripheral intravenous access program for emergency physicians, nurses, and corpsmen (technicians) at a military hospital. Mil Med. 2016;181(3):272-6. DOI: http://doi.org/10.7205/MILMED-D-15-00056 [ Links ]
9.Zingg W, Pittet D. Peripheral venous catheters: an under-evaluated problem. Int J Antimicrob Agents. 2009;34(Suppl 4):S38-42. DOI: http://doi.org/10.1016/S0924-8579(09)70565-5 [ Links ]
10.Egan G, Healy D, O'Neill H, Clarke-Moloney M, Grace PA, Walsh SR. Ultrasound guidance for difficult peripheral venous access: systematic review and meta-analysis. Emerg Med J. 2013;30:521-6. DOI: http://doi.org/10.1136/emermed-2012-201652 [ Links ]
11.Bauman M, Braude D, Crandall C. Ultrasound-guidance vs. standard technique in difficult vascular access patients by ED technicians. Am J Emerg Med. 2009;27(2):135-40. DOI: http://doi.org/10.1016/j.ajem.2008.02.005 [ Links ]
12.Stolz LA, Stolz U, Howe C, Farrell IJ, Adhikari S. Ultrasound-guided peripheral venous access: a meta-analysis and systematic review. J Vasc Access. 2015;16(4):321-6. DOI: http://doi.org/10.5301/jva.5000346 [ Links ]
13.Witting MD. IV access difficulty: incidence and delays in an urban emergency department. J Emerg Med. 2012;42:483-7. DOI: http://doi.org/10.1016/j.jemermed.2011.07.030 [ Links ]
14.Shokoohi H, Boniface K, McCarthy M, KhedirAl-tiae T, Sattarian M, Ding R, et al. Ultrasound-guided peripheral intravenous access program is associated with a marked reduction in central venous catheter use in noncritically ill emergency department patients. Ann Emerg Med. 201 ;61(2):198-203. DOI: http://doi.org/10.1016/j.annemergmed.2012.09.016 [ Links ]
15.Gregg SC, Murthi SB, Sisley AC, Stein DM, Scalea TM. Ultrasound - guided peripheral intravenous access in the intensive care unit. J Crit Care. 2010;25(3):514-9. DOI: http://doi.org/10.1016/j.jcrc.2009.09.003 [ Links ]
16.Dargin JM, Rebholz CM, Lowenstein RA, Mitchell PM, Feldman JA. Ultrasonography-guided peripheral intravenous catheter survival in ED patients with difficult access. Am J Emerg Med. 2010;28:1-7. DOI: http://doi.org/10.1016/j.ajem.2008.09.001 [ Links ]
17.Miranda RB, Nardino EP, Gomes T, Farias P. Nova técnica para treinamento em acessos vasculares guiados por ultrassom utilizando modelo de tecido animal. J Vas Bras. 2012;11(1):83-7. DOI: http://dx.doi.org/10.1590/S1677-54492012000100015 [ Links ]
18.Singer J. A simple procedure to compute the sample size needed to compare two Independent groups when the population variances are unequal. Stat Med. 2001;20(7):1089-95. DOI: https://doi.org/10.1002/sim.722 [ Links ]
19.Armitage P, Berry G. The planning of a statistical investigations. In: Armitage P, Berry G. Statistical methods in medical research. 2nd. Oxford: Blackwell; 1987; p. 179-85. [ Links ]
20.Gopalasingam N, Thomsen AE, Folkersen L, Juhl-Olsen P, Sloth E. A successful model to learn and implement ultrasound-guided venous catheterization in apheresis. J Clin Apher. 2017;32:437-43. DOI: https://doi.org/10.1002/jca.21533 [ Links ]
21.Brannam L, Blaivas M, Lyon M, Flake M. Emergency nurses' utilization of ultrasound guidance for placement of peripheral intravenous lines in difficult-access patients. Acad Emerg Med. 2004;11(12):1361-3. DOI: https://doi.org/10.1197/j.aem.2004.08.027 [ Links ]
22.Salleras-Duran L, Fuentes-Pumarola C, Bosch-Borràs N, Punset-Font X, Sampol-Granes FX. Ultrasound-guided peripheral venous catheterization in emergency services. J Emerg Nurs. 2016;42(4):338-43. DOI: https://doi.org/10.1016/j.jen.2015.11.005 [ Links ]
23.Sou V, McManus C, Mifflin N, Frost SA, Ale J, Alexandrou E. A clinical pathway for the management of difficult venous access. BMC Nurs. 2017;16;64. DOI: https://doi.org/10.1186/s12912-017-0261-z [ Links ]
24.Ueda K, Hussey P. Dynamic Ultrasound-guided short-axis needle tip navigation technique for facilitating cannulation of peripheral veins in obese patients. Anesth Analg. 2017;124(3):831-3. DOI: https://doi.org/10.1213/ANE.0000000000001653 [ Links ]
25.Mills CN, Liebmann O, Stone MB, Frazee BW. Ultrasonographically guided insertion of a 15-cm catheter into the deep brachial or basilica vein in patients with difficult intravenous access. Ann Emerg Med. 2007;50:68-72. DOI: https://doi.org/10.1016/j.annemergmed.2007.02.003 [ Links ]
26.Benkhadra M, Collignon M, Fournel I, Oeuvrard C, Rollin P, Perrin M, et al. Ultrasound guidance allows faster peripheral IV cannulation in children under 3 years of age with difficult venous access: a prospective randomized study. Paediatr Anaesth. 2012;22(5):449-54. DOI: https://doi.org/10.1111/j.1460-9592.2012.03830.x [ Links ]
27.Witting MD, Schenkel SM, Lawner BJ, Euerle BD. Effects of vein width and depth on ultrasound-guided peripheral intravenous success rates. J Emerg Med. 2010;39(1):70-5. DOI: https://doi.org/10.1016/j.jemermed.2009.01.003 [ Links ]
28.Panebianco NL, Fredette JM, Szyld D, Sagalyn EB, Pines JM, Dean AJ. What you see (sonographically) is what you get: vein and patient characteristics associated with successful ultrasound-guided peripheral intravenous placement in patients with difficult access. Acad Emerg Med. 2009;16(12):1298-303. DOI: https://doi.org/10.1111/j.1553-2712.2009.00520.x [ Links ]
29.Kerforne T, Petitpas F, Frasca D, Goudet V, Robert R, Mimoz O. Ultrasound-guided peripheral venous access in severely ill patients with suspected difficult vascular puncture. Chest. 2012;141(1):279-80. DOI: https://doi.org/10.1378/chest.11-2054 [ Links ]
30.Meyer P, Cronier P, Rousseau H, Vicaut E,Choukroun G, Chergui K, et al. Difficult peripheral venous access: clinical evaluation of a catheter inserted with the Seldinger method under ultrasound guidance. J Crit Care. 2014;29:823-7. DOI: https://doi.org/10.1016/j.jcrc.2014.04.022 [ Links ]
31.Doniger SJ, Ishimine P, Fox JC, Kanegaye JT. Randomized controlled trial of ultrasound-guided peripheral intravenous catheter placement versus traditional techniques in difficult-access pediatric patients. Pediatr Emerg Care. 2009;25(3):154-9. DOI: https://doi.org/10.1097/PEC.0b013e31819a8946 [ Links ]
32.Scoppettuolo G, Pittiruti M, Pitoni S, Dolcetti L, Emoli A, Mitidieri A, et al. Ultrasound-guided "short" midline catheters for difficult venous access in the emergency department: a retrospective analysis. Int J Emerg Med. 2016;9:3. DOI: https://doi.org/10.1186/s12245-016-0100-0 [ Links ]
33.Costantino TG, Parikh AK, Satz WA, Fojtik JP. Ultrasonography-guided peripheral intravenous access versus traditional approaches in patients with difficult intravenous access. Ann Emerg Med. 2005;46(5):456-61. DOI: https://doi.org/10.1016/j.annemergmed.2004.12.026 [ Links ]
34.Ault MJ, Rosen BT, Ault B. The use of tissue models for vascular access training. Phase I of the procedural patient safety initiative. J Gen Intern Med. 2006;21(5):514-7. DOI: https://doi.org/10.1111/j.1525-1497.2006.00440.x [ Links ]
35.Stein J, George B, River G, Hebig A, McDermott D. Ultrasonographically guided peripheral intravenous cannulation in emergency department patients with difficult intravenous access: a randomized trial. Ann Emerg Med. 2009;54(1):33-40. DOI: https://doi.org/10.1016/j.annemergmed.2008.07.048 [ Links ]
36.Nolting L, Hunt P, Cook T, Douglas B. An inexpensive and easy ultrasound phantom: a novel use for SPAM. J Ultrasound Med. 2016;35(4):819-22. DOI: https://doi.org/10.7863/ultra.14.06023 [ Links ]
37.Morrow DS, Broder J. Cost-effective, reusable, leak-resistant ultrasound-guided vascular access trainer. J Emerg Med. 2015;49(3):313-7. DOI: https://doi.org/10.1016/j.jemermed.2015.04.005 [ Links ]
38.Di Domenico S, Licausi M, Porcile E, Piaggio F, Troilo B, Centanaro M, et al. Introducing ultrasound-guided vein catheterization into clinical practice: a step-by-step guide for organizing a hands-on training program with inexpensive handmade models. J Ultrasound. 2008;11:135-42. DOI: https://doi.org/1016/j.jus.2008.09.002 [ Links ]
39.Hocking G, Hebard S, Mitchell CH. A review of the benefits and pitfalls of phantoms in ultrasound-guided regional anesthesia. Reg Anesth Pain Med. 2011;3692):162-70. [ Links ]
 Correspondence:
Correspondence:
ericapnardino@gmail.com
Manuscript received: November 2018
Manuscript accepted: May 2019
Version of record online: October 2019














