Journal of Human Growth and Development
ISSN 0104-1282
Rev. bras. crescimento desenvolv. hum. vol.22 no.3 São Paulo 2012
ORIGINAL RESEARCH
Iron deficiency during pubertal growth spurt
Carmen Lúcia de Almeida SantosI, II; Marco AkermanIII; Odival FaccendaIV; Lourdes Conceição MartinsV; Lígia de Fátima Nóbrega ReatoVI
IFaculdade de Ciências Médicas da Universidade Federal da Grande Dourados - MS
IIDisciplina de Pediatria. Faculdade de Ciências Médicas da Universidade Federal da Grande Dourados - MS
IIIDisciplina de Saúde Coletiva da Faculdade de Medicina do ABC, Santo André/SP
IVProfessor Titular. Universidade Estadual de Mato Grosso do Sul
VProfessora do Programa de Pós-Graduação em Saúde Coletiva da Universidade Católica de Santos - UNISANTOS
VIDisciplina de Hebiatria. Departamento de Pediatria. Faculdade de Medicina do ABC, Santo André/SP
ABSTRACT
INTRODUCTION: iron deficiency represents a serious injury to the health and it is associated with damages to the productive capacity of individuals, cognitive development and immune competence.
OBJECTIVE: verify the prevalence of iron deficiency in adolescents during the pubertal growth spurt.
METHODS: it was made a cross sectional study with clinical, laboratory and socioeconomic adolescents enrolled in Hebiatry Service of the Faculty of Medicine of ABC, in the city of Santo André, SP. Data were collected from 255 medical records containing complete medical history, including physical examination, classification of pubertal development (Tanner), weight, height, diet recall survey and the following laboratory tests: blood count, serum iron, transferrin saturation and stool tests.
RESULTS: of the 255 records studied, 162 (63.5%) were in the group of young people who were in the pubertal growth spurt and 93 (36.5%) belonged to the group who were outside the spurt. The presence of iron deficiency was higher among younger adolescents. There frequency of iron deficiency in 37 (14.5%), and 24 (16%) adolescents in the spurt and 13 (11.5%) out of the stretch. Regarding gender, it was found that of the 37 adolescents who had iron deficiency, 24 (64.46%) were males and 13 (35.14%) were females.
CONCLUSION: iron deficiency occurred more frequently in male adolescents during pubertal growth spurt and those who practiced sports.
Key words: adolescence; growth spurt; iron deficiency.
INTRODUCTION
World Health Organization defines adolescence as a period of human development characterized by the transition between their childhood and adult life with somatic, psychological and social changes. Puberty is understood when a fast physical growth is reached as there is a growth spurt peak besides a biological maturation (sexual and bone) 1.
Puberty arises by some development of secondary sex characteristics. External changes are the pubic hair in girls and boys, breast growth in girls and penis growth for boys. Inner changes are related to the gradual increase hormones rates produced by ovary in girls and testicular cells in boys2.
Data from National Health and Nutrition Examination Survey (NHANES- III), described by Frutuoso, Vigantzky and Gambardella3 are concerned to the effect of growth spurt, which occurs during the adolescence reflecting into the metabolism and iron deficiency. Teenagers present a higher demanding of these nutrients reasoned by the enlargement of total blood volume and iron pool, in consequence of an increase of thin body mass quantity. In addition, girls have a mineral loss due to the menstrual cycle. Thus, iron deficiency is nearly almost twice higher during puberty.
Most authors, among them, Fagioli and Nasser4 and Lima et al.5, believe during adolescence nutritional deficiency is more physiologically related to the age than chronologically as it is straight connected to the speed growth and the body change. For human body growing and keeping it balanced there are several chemical reactions which are determined by metabolism. Providing energy and taking part into the metabolic process are the roles of nutrients, including iron ingested through food in order to keep and maintain the health of the body.
Iron deficiency anemia is considered to be one of the main public health issues throughout the world. Insufficient dietary intake and absorption of iron is the top responsible for malnutrition. Even mildly, it is harmful to the healthy since it is associated to damages in productive capability, cognitive development and immune competence of individuals6.
Hence, the aim of this paper is to evaluate the connection between growth spurt and prevalence of iron deficiency in puberty.
METHODS
It is a retrospective, analytical descriptive and cross sectional study performed from July to December 2004. Medical reports data were assessed from 255 teenagers, aged 10 to 20 years old, who were enrolled in Teenagers first aid Unit from the Health School of ABC School of Medicine. They were split in two groups: male and female. Teenagers, who reported to have either any systemic pathology or were parasitoids carrier, were left out.
Field research is a primary health care service located in the suburb of the city Santo Andre, a municipality from the region of Grande São Paulo. Their policies are as following: scheduling appointment, enrollment and screening and then routine laboratorial exams are required: blood count, seric iron level (SIL), transferrin (TIBC), transferrin saturation (TSAT/TIBC)X100) and protoparasitology.
Samples were divided into two groups: teenagers who were already going through growing spurt and those who were not (control group). It was taken into account as growth spurt the boys who were met between the stage classification G3 and G4 (genital development) and for the girls M2 and M3 (breast development), according to Tanner's criteria. Nutritional evaluation was assessed by the Body Mass Index (BMI) whose value was compared to the NCHS frame of reference for each teenager.
Obtained results were compared to the preestablished standards. Hemoglobin (Hb)<11,5g/dL and hematocrit (Ht) < 35% for girls and boys along the initial period according to Tanner.
Along to the puberty growth spurt critical values were adopted as those advised by World Health Organization (WHO): Hb < 12,0 g/dL and Ht < 35% for girls and Hb < 12,5g/dL and Ht < 35%; seric iron < 40mcg/dL, TIBC>350mcg/dL for boys. Transferrin saturation was used < 16% e 20% respectively for women and men7.
Variables considered were: age, sex, menarche, nutritional classification, practice of sport, puberty development, menarche age, symptoms and anemia indication besides housing type condition and household income. As response variable was taken iron deficiency by using transferrin saturation.
For verifying association between two absolute variables it was taken either Pearson's chi-squared test (÷2) or Fischer exact test in cases there were an expected frequency lower than two, or whenever more than 20% of expected frequencies were lower than five.
Analysis which included a continuous response variable (transferrin saturation percentage) was used t-Student test in order to compare means differences. Variance analysis was used since there was more than two means involved to verify significant differences while Turkey's test was used two by two for multiple means comparisons. Parametric test was chosen reasoned to the variable transferrin saturation in which normal assumptions (Kolmogorov-Smirnov test) and equality between variance (Levene test) were attained.
A multi-variable test by applying logistic regression was performed to verify whether the association among the studied variables was on their own or there was any interference from a third part. All results were analyzed by taking into account the value p < 0,05 as a meaningful difference.
This paper was approved by the Ethics Committee in Research from Medical School of ABC (Nº 003/2006).
RESULTS
From those 255 medical records which were studied, 162 (63,5%) were from young teenagers who were going through puberty growth spurt and 93 (36,5%) belonged to those ones who were not.
Table 1 consists on the distribution according to the age. It was noticed that 10 to 13-year-old girls were among the larger sample group: 119, 46,7% of total, followed by 101 girls ranged 13 to 16 years old and 35 older than those (13,7%).
As it can be seen on Table 1, iron deficiency was higher among younger teenagers and decreasing proportionally among older ones. At the first age group was found 20 with iron deficiency (16,8%), decreasing to 14 (13,9%) and then 3 (8,6%) at the third group, but these differences were not statically significant (χ2(2) = 1,535, p = 0,464).
Regarding to the frequency of iron deficiency on the total sample it was found 37 (14,5%): 24 (16%) in puberty growth spurt and 13 (11,5%) who were out of it. Therefore, the difference between teenagers with and without iron deficiency was 4,5%. This difference was not statically significant (χ2(2) = 0,849; p = 0,357), that is, individuals among those two samples did not differ regarding to iron deficiency.
Regarding to sex, it was observed that from those 37 teenagers with iron deficiency 24 (64,46%) were male and 13 (35,14%) female. This difference was not statically significant (χ2 = 3,529; p = 0,060). On the other hand, taking into account only teenagers from puberty growth spurt, this difference is higher: 26 presented iron deficiency, 17 (65,4%) were male. That difference means a borderline difference (χ2 = 3,686; p = 0,055), although it is insufficient for rejecting hypothesis that an iron deficiency is equal for both sexes.
From those 91 young sport practitioners, 16 (17,6%) presented iron deficiency and from those 134 nonsport practitioners 15 (10%) presented iron deficiency. Yet, this difference is not high enough and it did not present a significant difference as well (χ2(1) = 2,837; p = 0,092). However, it was noticeable an iron deficiency trend among puberty growth spurt teenagers who were sport practitioners.
Data displayed on Table 2 shows that from the total studied teenagers, 197 (77,2%) were well-nourished, 18 (7,1%) obese, 25 (9,8%) thin and 15 (5,9%) were overweighed. Analyzing each group of teenagers individually who were in and out of growth spurt it was observed that from 127 well-nourished teenagers in growth spurt 19 (14,96%) had an iron deficiency diagnosis. From those 70 well-nourished teenagers, who were out of growth spurt, 6 (8,57%) had an iron deficiency diagnosis.
From those 129 male teenagers, 78 (60,5%) presented puberty growth spurt and 51 (39,5%) did not. Among male teenagers in puberty growth spurt 17 (21,8%) had iron deficiency. G3 stage group presented a higher percentage of iron deficiency: 11 (23,39%) (χ2(1) = 1,330; p = 0,249). However, this difference was lower and consequently, there was no significant difference (p > 0,05) (Table 3).
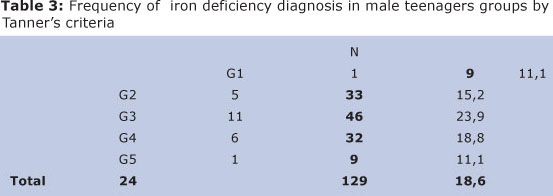
Among girls, 9 (10,7%) presented iron deficiency diagnosis and were going through growth spurt. 4 (9,5%) were met out of it. Stage M4 was the one in which presented a higher iron deficiency percentage, (16,7%), opposite to (9,7%) from those who did not belong to this stage (Table 4).
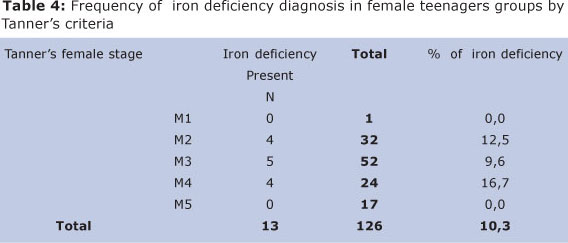
Other stages following Tanner's criteria for female teenagers, which this difference decreased, were statistically significant (p > 0,05).
Sample teenagers who reached menarche were a total of 66. From whom, 6 (9,1%) were identified with iron deficiency. Nevertheless, those who did not reach menarche, 6 (10,91%) presented iron deficiency. Thus, there was no significant deficiency, (χ2(1) = 0,223; p = 0,636), that is, iron deficiency in female teenager samples was indifferent whether they reached menarche. This is illustrated on Figure 1 by using tranferrin saturation value whereas the mean percentage of tranferrin saturation was not significantly different (t(114)= 0,595; p= 0,553) between menarche and non menarche, by Students t-test.
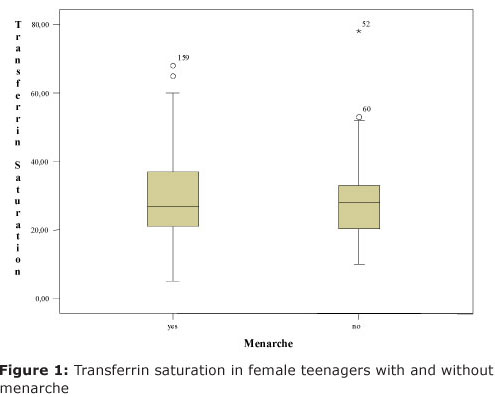
By variance analysis was noticed that the mean percentage of transferrin saturation in teenagers with menarche was significantly different between (F(2;62) = 9,72; p = 0,001) among distinct age groups. This mean percentage between groups at the ages 10 to 12 and 11 months was 21,37 (dp=6,2), which is significantly lower by Tukey's test from the one presented by other two age group female teenagers: between 13 to 15 yers and 11 months 36,17 (dp=16,74) and between 16 to 19 years and 11 months 34,18 (dp=9,48).
Yet, the mean percentage of transferrin saturation at the first age group had been statistically lower than the other two, iron deficiency diagnosis data did not present a significant conclusion (p = 0,392), that is, there was no association between iron deficiency and teenagers with menarche.
Regarding to housing style (bricklaying or wood) data did not present significant association between them and iron deficiency in teenagers (χ2(1) = 0,179; p= 0,672), neither with basic sanitation facilities (having or not) (χ2(1) = 1,307; p = 0,253), nor related to household income (below R$ 1.000,00; over R$ 1.000,00) (χ2(1) = 3,713; p = 0,054).
DISCUSSION
Puberty, period that occurs sexual maturation, demands a higher level of iron intake. Body grows faster than usual, reaching 8 to 12 cm a year for female and male, respectively. This growing period is named "puberty growth spurt". Along this time teenagers grow 20 cm in average, there may be a fluctuation from 10 to 30 cm, though.
Sex difference is noticed by muscle growth rates which are four-fold greater for male and twice for girls, according to Maddaleno8. Also, there is a higher production level of hemoglobin and RBC increase caused by stimulation of bone marrow and activation of carbonic anhydrase, directly exerted by testosterone. That is why teenagers may intake around 350 mg/ Fe/year, due to all these events.
Blood losses after menarche can also trigger an iron deficiency, which may be strengthened by irregular menstrual cycles since they are often unsteady in the first two years after menarche, despite normal nutritional offering. Blood loss is considered the second highest cause for iron deficiency, according to Fujimori et al.9 Diet must be well balanced throughout adolescence on account of previous factors mentioned above besides basic losses, which are approximately 14 gg/ Kg/day10.
Poor diet throughout adolescence may be caused by a variability of factors, including emotional instability, an obsessive craving for being thinner, general uncertain life style and social circumstances.
National Health and Nutrition Examination Survey - NHANES III shows that in a typical American diet, 27% of total daily energy come from high-energy food and are poor in nutrients (junk foods)11.
It has been noticed an increase of energy density on diet in Brazil, as it is showed by comparisons of food consume surveys performed in the country in 1961-62 (Fundação Getúlio Vargas 1970)12, in 1987-88 (IBGE - Instituto Brasileiro de Geografia e Estatística)13 and in 2008-09 (IBGE - Instituto Brasileiro de Geografia e Estatística/POF- Pesquisa de Orçamento Familiar, 2011)14. This latter one shows that among teenagers there has been a higher consume of sugar and cholesterol. Vitamin C and red meat, which instigate the increase of iron absorption of non heme iron displayed on the surveys as poor consume. This is due to the price of food that is higher and higher besides most Brazilian have not enough family income to purchase them.
Also, it was noticed that iron deficiency has not been an appropriation of none of the distributed groups in conformity of growth spurt. It has been presented in any adolescence stage, with a prevalence thought to be high (14,5%) whenever compared to results in other studies by Bruneira e Castro15, which reveal around 10% of prevalence for male teenagers and 5% for female ones. Yet sex differences have not been statistically significant, it was noticed a strong evidence that iron deficiency is more often in boys, being higher on those who were going through growth spurt, similar result as it is shown by Silva16. Likewise, obtained data from the study performed by Silva et al.17 throughout the assessment of anemia stemmed from iron deficiency during puberal growth spurt, show higher nutritional deficiency among male teenagers (50%) at genital development and among female teenagers (30%) at breast development. Furthermore, data from the study performed by Iuliano et al.18, with teenage students in the State São Paulo regarding to the incidence of anemia from iron deficiency, taking into account sexual maturation, it has been noticed a mild level of anemia (11%) among those individuals who were evaluated. This result caused the authors to conclude there is an increase of hemoglobin level along with sexual development among teenagers.
The study done by Garanito et al.19, opposite to some previous findings, reveals that in some countries, such as Switzerland, India and Indonesia iron deficiency is more often among female teenagers. Similarly, some statistics findings from a survey done by Vitalle e Fisberg20 show there is a higher incidence of iron deficiency among female teenagers in Pakistan, Ireland, United Kingdom and Mexico. In Brazil, several regional studies highlights teenage girls as having higher levels of iron deficiency. Equally, Vitalle and Fisberg20 show teenage girls are more prone to iron deficiency due to menstrual irregularities during the period after menarche, as they have abundant bleeding20. Unlikely, some European countries as Spain, Sweden and England, statistics do not show anemia among their teenagers.
In Brazil, Mariath et al.21 performed a research with children and teenagers assisted by a family health program in the city of Itajaí, State of Santa Catarina, found that statistics differences were not significant among aspects related to hemoglobin concentration, hematocrit and seric iron.
In Londrina, state of Paraná, Miglioranza et al.22 studied prevalence of anemia among teenage students from outskirts of the city, and results showed a percentage of 41,3% iron deficiency, having no significant difference between sexes besides there was no evidence the relations of iron deficiency and malnutrition.
Another study done in Salvador, State of Bahia, with teenage students from public schools, showed on the hemoglobin test that 24,5% of them revealed iron deficiency among female teenagers as minimum hemoglobin levels were 7,7g/dL23.
Even there has not been done a multicenter and national study in Brazil concerning to iron deficiency among teenagers, researchers share a common sense that iron deficiency is high among them. There is an estimate number of 20% without a sex classification once there is no official data13.
Furthermore, studies showing a higher prevalence among females have basic factors associated to the menarche, sexual maturation and growth spurt. In this way Azevedo et al.24 highlight that in adolescence the female body goes through fast changes related to its development as well as to sexual maturation. During menarche period occurs a natural mineral loss and consequently an iron deficiency anemia.
Since symptoms and indicators were rather insignificant it can be inferred iron deficiency is oligosymptomatic or asymptomatic. Thus, it makes it difficult to reach a clinic diagnosis, although it has been considered a pathology which attacks the health of teenagers and also persists as a public health problem. This is the reason Vitolo25 and Nead et al. 26 state that iron deficiency is a serious health issue, even if there is no prognosis
Vitalle and Fisberg13 explain that symptoms of anemia during adolescence are very subtle since they are frequently unnoticed. It is an insidious and late indication of iron deficiency caused by a negative balance of mineral27. However, some signs are quite notable such as tiredness, weakness and weight loss. Like this, Almeida28 says that, although it is almost imperceptible, iron deficiency shows a clinical case of "coetaneous mucosa paleness, asthenia, fatigue, feeding disorder and diminish of growing" besides the oxygen transport which is the main impairment due to the decreasing of hemoglobin concentration.
Therefore, there is an urge of laboratorial assessments for iron deficiency diagnosis in teenagers. Even they have no clear symptoms, other biochemical abnormalities may happen since iron take part into enzymatic reaction in the body. Consequently, there will be negative consequences in physical development and cognitive development, diminishing social integration opportunities of iron deficiency in individuals.
REFERENCES
1. Organização Mundial de Saúde. Status físico: o uso e a interpretação da antropometria. Genebra, 1995. (Relatório de Série Técnica, N. 854). [ Links ]
2. Mussen PH, Conger JJ, Kagan J, Huston AC. Desenvolvimento e personalidade da criança. 3.ed. São Paulo: Harbra: 2001. [ Links ]
3. Frutuoso MFP, Vigantzky VA, Gambardella AMD. Níveis séricos de hemoglobina em adolescentes segundo estágio de maturação sexual. R. Nutr 2003;16 (2):4-11. [ Links ]
4. Fagioli D, Nasser LA. Educação nutricional na infância e na adolescência: planejamento, intervenção, avaliação e dinâmicas. São Paulo: RCN: 2008. [ Links ]
5. Lima ACVM, Lima MC, Guerra MQF, Romani SAM, Eickamnn SH, Lira, PIC. Impacto do tratamento semanal com sulfato ferroso sobre o nível de hemoglobina, moribade e estado nutricional de lactantes anêmicos. Jornal de Pediatria. 2006: 82 (6): 452-57. [ Links ]
6. Batista Filho M, Rissin A. A transição nutricional no Brasil: tendências regionais e temporais. Caderno de Saúde Públlica 2003; 19(1): 181-191. [ Links ]
7. Coates V, Beznos GW, Françoso LA. Medicina do Adolescente. Ed.Sarvier 2ª edição. São Paulo: 2003, p.241-245. [ Links ]
8. Maddaleno M. La salud del adolescente y del joven. Washington: Organización Panamericana de la Salud: 1995, p.87-93. [ Links ]
9. Fujimori E, Szarfarc SC, Oliveira IMV. Prevalência de anemia e deficiência de ferro em adolescentes do sexo feminino - Taboão da Serra, SP, Brasil. Rev. latino-am. enfermagem, Ribeirão Preto, 1996; 4(3): 49-63. [ Links ]
10. Mahan KL, Escott-Stump S. Nutrição na adolescência. In: Mahan KL, Escott-Stump S. Krause - Alimentos, Nutrição e Dietoterapia. 11.ed. São Paulo: Roca; 1998. p.279-83. [ Links ]
11. Kant AK. Consumption of energy-dense, nutrient-poor foods by adult Americans: nutritional and health implications. The Third National Health and Nutrition Examination Survey, 1988-1994. Am J Clin Nut, 2000;72 (2): 929-36. [ Links ]
12. Fundação Getúlio Vargas. Consumo de alimentos no Brasil: pesquisa de orçamento familiar (1961-62). Rio de Janeiro: FGV, 1970. [ Links ]
13. IBGE - Instituto Brasileiro de Geografia e Estatística. POF Pesquisa de Orçamento Familiar (1987-88). Tabelas de Composição Nutricional dos Alimentos Consumidos no Brasil. Rio de Janeiro: IBGE, 1991, p.228-239. [ Links ]
14. IBGE - POF - Pesquisa de Orçamentos Familiares (2008-09). Tabelas de Composição Nutricional dos Alimentos Consumidos no Brasil. Disponível em: http://www.ibge.gov.br/home/estatistica/populacao/condicaodevida/pof/2008_ 2009_composicaonutricional/default.shtm. Acessado em 08 de julho de 2011. [ Links ]
15. Bruneira P, Castro HC. Anemia ferropriva. In: Coates V, Beznos GW, Françoso LA. Medicina do Adolescente. Ed.Sarvier 2 ed. São Paulo: 2003, p.241-245. [ Links ]
16. Silva MC. Anemia por deficiência de ferro na adolescência. Núcleo de Estudos da Saúde da Adolescência - UERJ 2007; 4(1):19-22. [ Links ]
11. Silva FC, Vitalle MSS, Quaglia EC, Braga JAP, Medeiros EHGR. Proporção de anemia de acordo com o estadiamento puberal, segundo dois critérios diagnósticos. Revista de Nutrição 2007; 20(3): 297-306. [ Links ]
17. Iuliano BA, Frutuoso MFP, Gambardella AMD. Anemia em adolescentes segundo maturação sexual. Revista de Nutrição 2004; 17 (1): 37-43. [ Links ]
18. Garanito MP, Pitta TS, Carneiro JDA. Deficiência de ferro na adolescência. Revista Brasileira de Hematologia e Hemoterapia 2010; 32(2): 45-48. [ Links ]
19. Vitalle MSS, Fisberg M. Deficiência de ferro entre adolescentes. II Jornada de Anemia Carencial e Segurança Alimentar no Brasil. Jornadas Científicas do NISAN 2007/2008: 161-73. [ Links ]
20. Mariath AB, Giachini RM, Lauda LG, Grillo LP. Estado de ferro e retinol sérico entre crianças e adolescentes atendidos por equipe da Estratégia de Saúde da Família de Itajaí, Santa Catarina Ciência e Saúde Coletiva 2010; 15 ( 2): 509-516. [ Links ]
21. Miglioranza LHS, Matsuo T, Caballero-Córdoba GM, Dichi JB, Cyrino ES, Oliveira IBN, Martins MS, Polezer N, Dichi I. Anemia prevalence in children and adolescents from educational centers in the outskirts of Londrina, PR, Brazil. Revista de Nutrição 2002;15(2): 149-53. [ Links ]
22. Borges CQ, Silva RCR, Assis AMO, Pinto EJ, Fiaccone RL, Pinheiro SMC. Fatores associados à anemia em crianças e adolescentes de escolas públicas de Salvador, Bahia, Brasil. Caderno de Saúde Pública 2009; 25 (4):877-88. [ Links ]
23. Azevedo L, Martino HSD, Carvalho FG, Rezende, ML. Estimativa da ingestão de ferro e vitamina C em adolescentes no ciclo menstrual. Revista Ciência e Saúde Coletiva 2010; 15(1): 1359-367. [ Links ]
24. Vitolo MR. Nutrição: da gestação à adolescência. Rio de janeiro: Reichemann & Affonso Editores: 2003, p.193-6. [ Links ]
25. Nead KG, Halferman JS, Kaczorowski JM, Auinger P, Weitzman M. Overweight children and adolescents: a risk group for iron deficiency. Pediatrics 2004; 134(1):104-08. [ Links ]
26. Beard JL. Iron requirements in adolescent females. American Socity for Nuttitional Sciences 2000; 440S-43S. [ Links ]
27. Almeida E. Anemia ferropriva: como diagnosticar? Lincx 201. Disponível em http://www.lableme.com.br. Acessado em 08 de julho de 2011. [ Links ]
 Corresponding author:
Corresponding author:
carmen.elias@globo.com
Manuscript submitted Jul 16 2011
Accepted for publication Aug 30 2012













