Revista de Etologia
ISSN 1517-2805 ISSN 2175-3636
Rev. etol. v.6 n.1 São Paulo jun. 2004
DOSSIER
Thigmotaxis and exploration in adult and pup rats*
Tigmotatismo e exploração em ratos adultos e filhotes
Raquel Martinez; Silvio Morato
Universidade de São Paulo, Faculdade de Filosofia, Ribeirão Preto
ABSTRACT
Rats tend to investigate novel environments and objects present in such environments. Exploratory behavior seems to be limited by thigmotaxis, a tendency to remain close to vertical surfaces the probable function of which is to protect rats from predation. Our studies show that the more circumscribed the place, the stronger the attraction it exerts. They also show that thigmotaxis is a very strong response either in individual or grouped rats. Motivation to explore a novel environment appears around the 21st day of life, three or four days after the rats' eyes open, but even at this early age, pups are responsive to thigmotaxis stimuli. Thigmotaxis may thus be an important defensive mechanism which appears precociously in rat development.
Index terms: Exploratory behavior, Anxiety, Thigmotaxis, Open-field, Rats.
RESUMO
Ratos expostos a ambientes não familiares tendem a explorar o local e os objetos nele presentes. O comportamento exploratório parece ser limitado pelo tigmotatismo, a tendência a permanecer perto de superfícies verticais que provavelmente tem por função proteger os ratos de predadores. Nossos estudos mostram que quanto mais circunscrito um local, maior a atração que ele exerce sobre os animais. Mostram também que os ratos, quer estejam sozinhos, quer em grupo, sempre exibem tigmotatismo. O comportamento exploratório exibido em ambientes não familiares surge por volta do 21º dia de vida, três ou quatro dias depois da abertura dos olhos, mas mesmo nessa pouca idade os filhotes respondem com tigmotatismo. O tigmotatismo, que aparece cedo na vida dos ratos, pode constituir um importante mecanismo de defesa.
Descritores: Comportamento exploratório, Ansiedade, Tigmotatismo, Campo aberto, Ratos.
Some animals species remain in a certain area during their lifetime and display, in this area, exploratory behavior, an spontaneous expression of their curiosity (Renner and Seltzer, 1991). Home range varies according to the animal's needs and availability of resources (Barnett, 1975). Rats are territorial animals which use their territory to get food, water, nest material and sexual mates, being guided in their activities by environmental cues (Fowler, 1966). They also enter into unfamiliar environments, and display exploratory behaviors elicited by novelty (Berlyne, 1950). A general theory of behavior has to take into account curiosity and exploration as a fundamental part of the behavioral repertoire of animals.
When an adult rat gains access to a new environment the amount and intensity of exploratory behavior is related to the amount of similarity of such environment to familiar ones (Montgomery, 1955). When rats are re-exposed to a site, they tend to explore more the region they explored less in the first time (Barnett, 1958). Exploratory behavior can be elicited when an animal enters a new area and when unfamiliar stimuli occur in an already known environment (Barnett, 1958, Berlyne, 1955).
Studies of space use in the laboratory are usually performed by placing animals in closed environments such as mazes or open-fields. Exploration is then not spontaneous, once the animals do not get into such environments by themselves (Welker, 1957). An important determinant of the rat's behavior, in these tests, seems to be thigmotaxis, the tendency to remain close to vertical surfaces (Treit and Fundytus, 1989) possibly as a protection against aerial predation (Grossen e Kelly, 1972). How the rats detect vertical surfaces and what environmental stimulus trigger this behavior still remains a problem to be solved. Schiffman et al. (1970) raised the hypothesis that vision could eventually be the most relevant sensory system used by rodents to obtain this information. Animals could, alternatively, use their vibrissae to detect vertical surfaces and walls, a behavior which could be advantageous in the dark. Cardenas et al. (2001), however, provided evidence that rats may not use their vibrissae when exploring a novel environment.
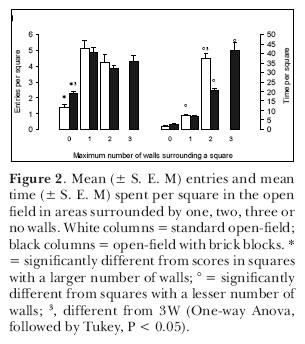
We attempted to assess the influence of thigmotaxis on the exploratory behavior of rats, leaving open the question of its sensory basis. In a first experiment, we examined the hypothesis that rats avoid the open arms of an elevated plus-maze because, in such places, they cannot stay close to vertical surfaces (Treit and Fundytus,1989). This hypothesis might explain why circumscribed zones (which constitute a somewhat closed space) attract rats. In order to test it, we placed adult male rats in a square open-field and recorded their position as a function of the number of circumscribing walls. One group of animals was observed in a common open-field; another one was observed in an open-field with two blocks in two of the corners (Figure 1). In the latter apparatus, rats could stay in areas circumscribed by one, two, three or no walls as compared to a maximum of two in the first one. The results indicated that the rats entered more into the squares that had at least one wall; but that they remained longer in areas circumscribed by two or, when available, three walls (Figure 2). When they had squares circumscribed by a maximum of two walls they spent most of their time there; when they were circumscribed by a maximum of three walls that was where the rats spent most of their time. If given the choice, young adult rats prefer to stay in places surrounded by the largest possible number of wall in a given environment, thus confirming the thigmotaxis hypothesis.
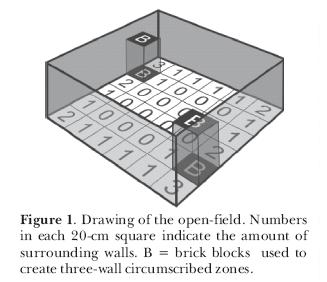
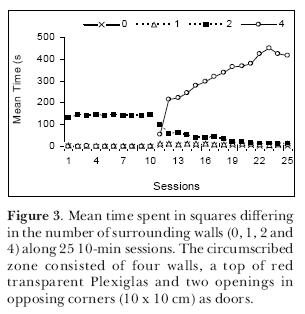
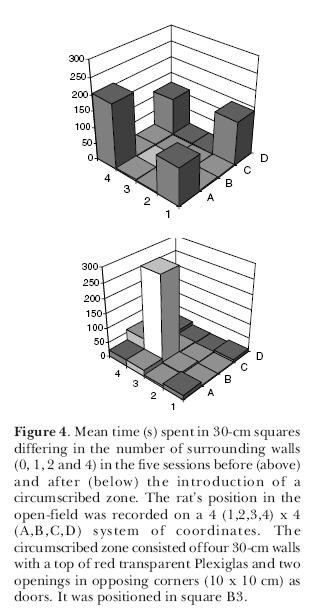
To further explore the matter, we performed another experiment (Joseane Bomfim carried out the sessions) again testing young adult male Wistar rats in the open-field but this time, instead of using blocks, we circumscribed a zone using four wooden walls that formed a square enclosure (40 x 40 x 40 cm) with a ceiling of red transparent Plexiglas and small entrances (10 x 10 cm) in two opposed corners at the floor level. The open-field was divided into 40-cm squares and instead of testing each rat only once, we submitted 2-month old rats to 25 10-min sessions in this apparatus. In the first 10 sessions, the rats freely explored the open-field without the circumscribed zone; from the 11th day until the end, the enclosure was present. Results are expressed in Figure 3. It can be seen that in the first 10 sessions, the rats behaved like the ones in the first experiment and preferred to remain in the corners circumscribed by two walls. In the sessions with the circumscribed zone, the rats gradually spent more time in it and during the last sessions they remained there almost all the time (Figure 4; notice the slightly increased use of the zone formed between the circumscribed zone and one of the walls). We once again confirmed the thigmotaxis hypothesis. It seems, however, that if the circumscribed zone is totally closed (four walls with a ceiling and entrances), the rats are at first more cautious to occupy it.
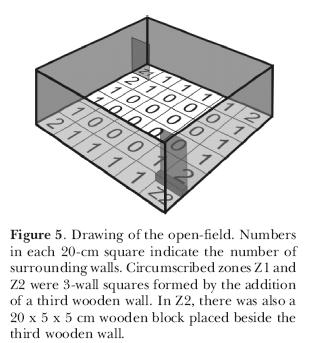
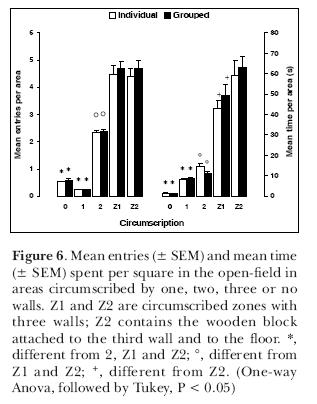
In another series of experiments, we investigated whether this pattern of exploration would still be maintained if male rats were tested individually or in groups of six at once in the open-field. Animals were placed in the open-field, with two opposed corners circumscribed by a third wall (Z1 and Z2; Figure 5). The difference between one and the other was the presence of a small wooden block 5-cm high and attached to the third wall and to the floor. Results (Figure 6) indicate there were no differences between animals tested alone and in groups of six. There were however differences in the use of the areas. Areas with no walls or just one wall were less explored, either in terms of frequency of entries or in terms of time. Areas with two walls were entered more than one-wall or no wall areas but not as many times as the circumscribed zones Z1 and Z2. The time spent in the latter was significantly higher than the areas with two, one or no walls. This shows that rats have the same behavior, whether in groups or singly tested. The differential occupation of the areas favors again the thigmotaxis hypothesis.
We then focussed on the beginning of exploration in male rat pups. We used a cage with a sliding door small enough for the pups to leave the cage but not sufficiently large for dams to pass through it. Dams, each with six one-day pups, spent 30 days in this cage. From the 10th day on, the cage was placed in the center of the open-field for 30 minutes with the sliding door open. This experiment was performed twice. From the 23rd day on, the pups started leaving the cage and exploring its surroundings in the open-field. In fact, from the 21st day on, upon opening the sliding door, the pups attempted to leave the cage but were prevented by the mother that placed herself with her back to the sliding door and pushed every pup who approached it while the door was open. That behavior was not observed when the sliding door was closed.
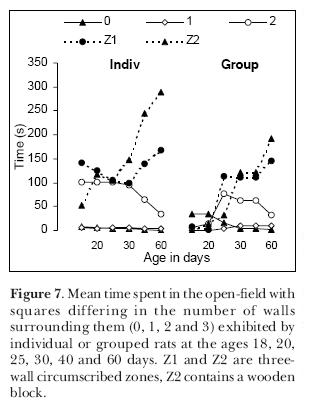
Finally, we repeated the experiment with individual or grouped male animals but testing the same animals more than once. We tested the pups at days 18, 20, 25, 30, 40 and 60 of age. We chose to start the tests on day18, because the eyes usually open between the 16th and 17th days. The main difference between individual and grouped pups was that the latter, on days 18 and 20, tended to remain in the place they were placed in the open-field and exhibit no exploratory behavior at all (Figure 7). From the 25th day on, all pups behaved alike and tended to seek, as young adult rats do, the more protected circumscribed zones Z1 and Z2.
Unlike young adults, however, pups exhibited play behavior when tested in groups. Many different hypotheses have been advanced concerning the functions of play in different species such as the physical exercise hypothesis (Brownlee, 1954), or various types of socialization and acquisition of social skills hypotheses (Poirier and Smith, 1974) and even the learning of mothering skills (Lancaster, 1971). The most frequently play behavior we observed was ambulation in the open-field in a tandem formation; the animals varied their positions and no leader could be observed in the tandems. Beginning with day 25, this behavior appeared with low frequency, involving at most two or three pups. Between days 30 and 40, play behavior was very frequent and involved from three to six animals. Play is conceived to be an activity of young, developing animals rather than of adults and is believed to be important as a basis for adult behavior (Martin and Caro, 1985). Our results support existing data by showing that tandem play behavior in the rat has an inverted U relationship to age, with a peak at 30-40 days of age (Meaney and Stuart, 1981). Tandem play was the predominant form of play we observed in our rats; other forms of play are mentioned in other studies: pinning (one of the animals lies with dorsal surface on the floor while the other stands over it), boxing/wrestling and following/chasing (Vanderschuren et. al. 1995, 1997).
All our results show that male rats of different ages entered more frequently and stayed longer in the squares or areas with the largest number of walls, as compared to the squares with less walls around. Young adult rats explore a novel environment but spend more time and effort seeking protection in more structured circumscribed zones, obeying to the thigmotropic tendency; i. e., they always show a preference for remaining in areas with the largest possible number of walls available. Very young rat pups however do not display this behavior. We can also conclude, although our data are just descriptive, that when they get older (mainly at 30-40 days of age) rats engage in play behavior in familiar environments, and that they do not play when reaching the age of young adults (60 days), as judged by the disappearance of tandem behavior at this age. We now have the standard exploratory behavior pattern of pups in familiar environments. The next step in our research will be to study the behavior of pups at different ages in the open-field in the absence or presence of their dams (in a separate cage but allowing the pups to see and smell her). Experiments such as these may allow us to similarities and differences between the behavior of rat pups and that of children in day care centers.
References
Barnett, S. A. (1956). Behaviour components in the feeding of wild and laboratory rats. Behaviour, 9, 24-43. [ Links ]
Barnett, S. A. (1958). Exploratory Behaviour. British Journal of Psychology, 49: 289-310. [ Links ]
Barnett, S. A. (1975). The Rat: A Study in Behavior. The University of Chicago Press, Chicago. [ Links ]
Berlyne, D. E. (1950). Novelty and curiosity as determinants of exploratory behavior. British Journal of Psychology, 41, 68-80. [ Links ]
Berlyne, D. E. (1955). The arousal and satiation of perceptual curiosity in the rat. Journal of Comparative Physiology Psychology, 48, 238-246. [ Links ]
Brownlee, A. (1954). Play in domestic cattle in Britain: an analysis of its nature. British Veterinary Journal. 110, 46-68. (Apud Martin and Caro, 1985). [ Links ]
Cardenas, F.; Lamprea, M. R. and Morato, S. (2001). Vibrissal sense is not the main sensory modality in the rat exploratory behavior in the elevated plus-maze. Behavioural Brain Research, 122, 169-174. [ Links ]
Fowler, H. (1966). Curiosity and Exploratory Behavior. The Critical Issues in Psychology Series. New York. [ Links ]
Grossen, N.E. and Kelly , M.J. (1972). Species-specific behavior and acquisition of avoidance behavior in rats. Journal of Comparative Physiological Psychology, 81, 307-310. [ Links ]
Lancaster, J. B. (1971). Play mothering: the relations between juvenile females and young infants among free-ranging vervet monkeys (Cercopithecus aethiops). Folia Primatologica, 15, 161-182. (Apud Martin and Caro, 1985). [ Links ]
Martin, P. and Caro, T. M. (1985). On the functions of play and its role in behavioral development. Advances in the Study of Behavior, 15, 59-103. [ Links ]
Meaney, M. J. and Stuart, J. (1981). A descriptive study of social development in the rat (Rattus norvegicus). Animal Behaviour, 29, 34-45. [ Links ]
Montgomery, K. C. (1955). The relation between fear induced by novel stimulation and exploratory drive. Journal of Comparative and Physiological Psychology, 48, 254-260. [ Links ]
Poirier, F. E. and Smith, E. O. (1974). Socializing functions of primate play. American Zoologist, 14, 275-287. [ Links ]
Renner, M. J. and Seltzer C. P.(1991). Molar characteristics of exploratory and investigatory behavior in the rat (Rattus novergicus). Journal of Comparative Psychology, 1051, 326-339. [ Links ]
Schiffman, H. R., Lore, R., Passafiume, J. & Neeb, R. (1970). Role of vibrissae for depth perception in the rat (Rattus novergicus). Animal Behavior, 18, 290-292. [ Links ]
Treit, D. and Fundytus, M. (1989). Thigmotaxis as a test for anxiolytic activity in rats. Pharmacology Biochemistry and Behavior, 31, 959-962. [ Links ]
Vanderschuren, L. J. M. J.; Niesink R. J. M.; Spruijt, B. M. and Van Ree, J. M. (1995). Influence of environmental factors on social play behavior of juvenile rats. Physiology and Behavior, 58, 119- 123. [ Links ]
Vanderschuren, L. J. M. J., Niesink, R. J. M. and Van Ree, J. M. (1997). The neurobiology of social play behavior in rats. Neuroscience and Biobehavioral Reviews, 31, 309-326. [ Links ]
Welker, W. I. (1957). `Free' versus `forced' exploration of a novel situation by rats. Psychological Reports, 3, 95-108. [ Links ]
 Correspondence
Correspondence
Silvio Moratodo
Faculdade de Filosofia,
Av. Bandeirantes, 3900
14040-901, Ribeirão Preto, SP, Brasil.
E-mail: smorato@ffclrp.usp.br
Received September 03, 2003
Revision received November 17, 2003
Accepted March, 2004
* Paper presented at the III Congresso Norte-Nordeste de Psicologia, João Pessoa, Brazil, 2003.














