Serviços Personalizados
Journal
artigo
Indicadores
Compartilhar
Revista de Etologia
versão impressa ISSN 1517-2805versão On-line ISSN 2175-3636
Rev. etol. v.8 n.1 São Paulo jun. 2006
ARTIGOS
Cephalopods and fish attracted by night lights in coastal shallow-waters, off southern Brazil, with the description of squid and fish behavior
Rodrigo Silvestre Martins1, 2; José Angel Alvarez Perez3
1 University of Cape Town - South Africa
2 Universidade do Vale do Itajaí, Santa Catarina
3 Centro de Ciências Tecnológicas da Terra e do Mar (CTTMar)
ABSTRACT
Squid and fish behaviour under light attraction were opportunistically registered during a study of the ecology of loliginid squid in shallow-waters, undertaken at ‘Pântano do Sul’ bight (27°47'18'' S; 48°31'07'' W), south of ‘Santa Catarina’ Island, southern Brazil, in three consecutive austral summers, between 1999 and 2001. Three species of squid (Loligo plei, L. sanpaulensis and Lolliguncula brevis) and 12 species of fish were attracted by the night-lights. Schools ofLoligo spp. squid were observed swimming in and out the lighted area. JuvenileL. plei and L. sanpaulensis were found to mix in a single school. L. brevis often remained under the lights, where they performed remarkable behaviors, which included agonistic body posture (‘flamboyant’) and mimicry of small clupeiform fish. Among fish, the cutlassfish (Trichiurus lepturus) presented an ambushing predatory behavior towards the schools of small clupeiform fish (anchovies, Anchoa spp. and sardines, Sardinella brasiliensis, Harengula clupeola) attracted by the night lights. An emphasis was given to the squid–fish interactions, which are described and discussed.
Keywords: Light attraction, Squid, Fish.
RESUMO
O comportamento de lulas e peixes sob atração luminosa foi oportunisticamente registrado em um estudo sobre a ecologia de lulas da família Loliginidae em águas razas na Enseada do Pântano do Sul (27°47'18'' S; 48°31'07'' W), sul da Ilha de Santa Catarina durante os verões de 1999, 2000 e 2001. Três espécies de lulas (Loligo plei, L. sanpaulensis e Lolliguncula brevis) e 12 de peixes foram atraídas pelas luzes de pesca e tiveram seus comportamentos registrados. Cardumes de lulas e peixes foram observados entrando e saindo da zona iluminada pelas luzes de pesca. Lulas pequenas de duas espécies (Loligo plei e L. sanpaulensis) foram registradas formando um único cardume. Lolliguncula brevisfoi a lula que mais tempo permaneceu sob as luzes, demonstrando comportamentos complexos, que incluíram posturas corporais agonísticas e mimentismo de pequenos peixes pelágicos. Dentre as espécies de peixe, os peixes-espada (Trichiurus lepturus) apresentaram comportamento predatório de emboscada em relação as lulas e cardumes pequenos clupeiformes (Anchoa spp., Sardinella brasiliensis, Harengula clupeola) atraídos pelas luzes. As interações entre lulas e peixes sob atração luminosa são descritas e discutidas.
Palavras-chave: Atração Luminosa, Lulas, Peixes.
Artisanal squid fishing in Brazil traditionally takes place during summer months along the southeast and southern coasts, between Rio de Janeiro (23°S) and Santa Catarina States (29°S) (Costa and Haimovici, 1990; Perez, Martins and Buratto, 1999; Martins, 2002). Several types of fishing gears are employed in this area, and nocturnal fishing is often carried out using light attraction. In spite of the widespread use of light attraction in squid fishing worldwide (Rathjen and Voss, 1987), and several accounts on the behaviour of squid attracted by night lights [synthesized by Hanlon, Hixon, Forsythe and Hendrix Jr (1979)], to date, no studies on this subject have been made in Brazil.
Nonetheless, advances in using light attraction methods have been proven to be very useful not only providing material for physiological and behavioural investigations not previously possible (Moltschaniwskyj and Doherty, 1995) but also allowing the direct field observation of the behaviour of the components of the pelagic community, mostly fish and squid (Woodhead, 1966; Hanlon et al., 1979). In addition, sampling with light attraction has also been used for the assessment of species composition, abundance and distribution patterns of juveniles of both cephalopod and fish (Thorrold, 1992; Moltschaniwskyj and Doherty, 1995).
Within this context, the aim of the present note was to describe the behaviour and community interactions involving juvenile and adult squid and fish under light attraction in shallow waters around Santa Catarina Island, southern Brazil, particularly from the perspective of squid predator–prey interactions.
Methods
An opportunistic study of the behaviour and dynamics of the components of the coastal community was conduced during night at ‘Pântano do Sul’ Bight (27°47'18'' S; 48°31'07'' W), south of Santa Catarina Island, southern Brazil. Sampling trips (n = 13) were carried out in a bi-weekly basis, in three consecutive austral summers (November–March), between 1999 and 2001 (Martins, Perez and Schettini, 2006 in press).
Components of the pelagic community (i.e., squid, fish, zooplankton) were concentrated around the boat during the night with light attraction. The lights consisted of two 60-watt fluorescent light bulbs attached to the tip of a stick fastened perpendicularly to the boat and feed by a 12 V car battery.
Samples of squid, fish, polychaetes and macrozooplankton present in the near-surface layer (up to ~ 50 cm) were taken with dipnets, stored in 4% buffered formalin and brought to the laboratory where they were identified to the lowest taxonomic level. Sizes were measured using mantle length (ML) for squid, total length (TL) for fish and the carapace length (CL) for crabs. All measurements were made to the nearest millimeter.
Observations on the behaviour of squid, fish and the other taxa attracted by the lights were made between the dusk and dawn (~ 19:00–06:00h local time), while squid jigging was ongoing (Martins et al, in press). Video footage was made from the surface with a VHSC video camera in 1999 season and the high water clarity allowed the observations to be made as deep as 5–7 meters.
Results and Discussion
A total of 557 specimens of loliginid squid, belonging for three species (Loligo plei, L. sanpaulensisand Lolliguncula brevis), 79 fish (11 species), 4 juveniles of swimming crab (Callinectes sp.), epitocal polychaetes and several decapod crustacean larvae (unidentified megalopae and mysis) were captured in the 3 seasons (Table 1).
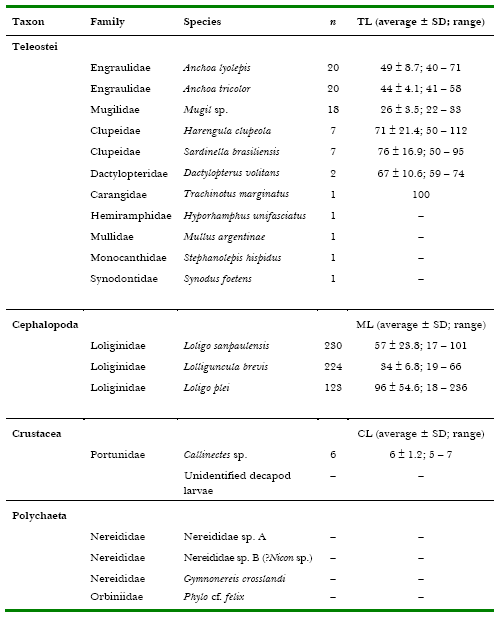
Table 1. Fish, squid, crustaceans and epitocal polychaetes caught with dipnets and lighting attraction at “Pântano do Sul” Bight, Santa Catarina Island, southern Brazil, in the summers of 1999, 2000 and 2001. n = number of individuals, SD = standard deviation, TL = total length (fish), ML = mantle length (squid), CL = carapace length (crabs). All measurements in millimetres.
In general, dense zooplankton concentrations were aggregated under the lights, but the densities of the swarms varied between sampling trips, with varying wind conditions (few or no zooplankton in southerly wind) and even time of the night. Usually, the zooplankton formed a layer in the first few centimeters of the water column nearest to surface, followed immediately underneath by schools of small pelagic fish (generally anchovies or sardines) and, below the fish, groups of squid schools and scattered individual large fish predators up to 2 m deep (Fig. 1A). The behaviors of squid and fish attracted by the lights are described below.
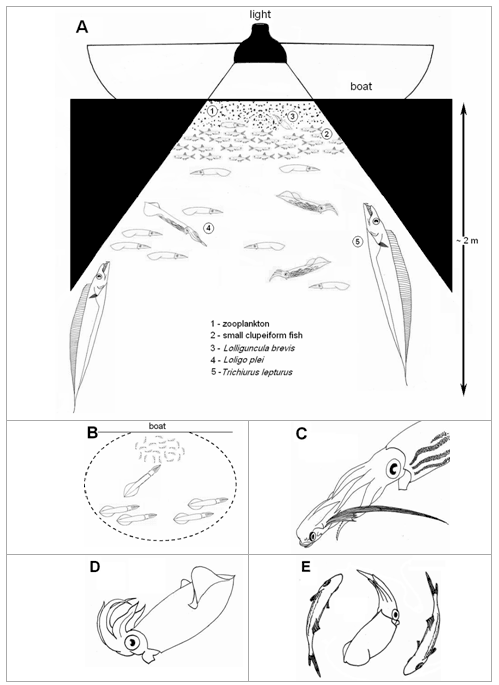
Figure 1. Pelagic organisms attracted to the night lights during sampling trips at “Pântano do Sul” Bight during summer, between 1999 and 2001. A) Schematic of the general structure and behaviour of the organisms. B) Predatory behaviour of Loligo plei towards schools of small clupeiform fish (the doted circle represents the lighted area). C) Loligo plei eating a flying gurnard (Dactylopterus volitans). D) Lolliguncula brevis showing a flamboyant display. E) Lolliguncula brevis mimicking small clupeiform fish (Anchoa spp.).

Figure 2. A small school of adult Loligo plei crossing the lighted area underneath the boat 5 meters deep at “Pântano do Sul” Bight, Santa Catarina Island, southern Brazil in 1999 summer.
Squid Behaviour
Three squid species were attracted to the night lights. Loligo sanpaulensis were the most frequent species, followed byLolliguncula brevis and L. plei (Table 1). In general, most of Loligo spp. swimming near the surface were juvenile (< 80 mm ML) and comprised mainly byL. sanpaulensis, while adult and sub-adult squid (> 80 mm ML; mostly L. plei) swam some 20–60 cm below the surface or deeper, only doing short excursions to the near surface to seize small fish or polychaetes (Fig. 1B). Squid of all sizes swam in and out the lighted area. TheLoligo spp. squid often swam in schools (i.e., group of same-sized individuals swimming in synchrony, sensu Hanlon and Messenger, 1996). School sizes ranged in number between 3 and 48 individuals in juvenile squid, and 3 to 6 in the adult and sub-adults (Fig. 2). AdultL. plei squid were often comprised by a large individual (supposedly a dominant male, given the conspicuous red-brownish banded coloration visible on each side of the mantle) and slight smaller ones, which were thought to be females, possibly comprising his ‘harem’ (Hanlon, Hixon and Hulet, 1983). This was consistently confirmed afterwards in the catches with dipnets.
L. plei were observed preying upon fish and epitocal polychaetes in the lighted area. Groups of squid were swimming tail-first in circles around the schools of small fish (approach phase) and, when they get very close (ca. 10–15 cm), the squid quickly darted forward and seized the fish (Fig. 1B), often in the central region of the body with the head and tail protruding from the squid’s arms (capture phase). The capture phase was very similar to the results reported for the ommastrephid squidIllex illecebrosus (Foyle and O’Dor, 1988). Some individuals caught with the dipnets still had small fish within the arm crown. These were usually anchovies (Anchoa spp.), although a flying gurnard (Dactylopterus volitans), of about 120 mm, was recovered from a 205 mm ML squid. The gurnard was found being devoured from behind, i.e., tail first (Fig. 1C). This suggests thatL. plei manipulate this fish species in order to avoid the sharp and robust cephalic bones of this species to be ingested and thus internal injuries (Collins and Pierce, 1996). Squid were seen attacking the polychaetes very few times, and in all occasions they released the worms shortly after the seizure. Remains of both fish and polychaetes were recorded in the stomach contents (Martins et al., 2006 , in press).
A mixed school of juvenileL. plei and L. sanpaulensis were caught at the night of 06-07 January 2001. However, the sizes of the species were different, withL. plei being larger than L. sanpaulensis (student’s t test, p < 0.01) (Fig. 3). Multi-species squid schools have been recorded previously in several localities, although mixing maybe of short duration. Different loliginid squid species can mix up within a single school, at least for short periods of time, but this behaviour was only known for adult squid (Moynihan and Rodaniche, 1982; Cohen, 1976; Hanlon et al., 1983).
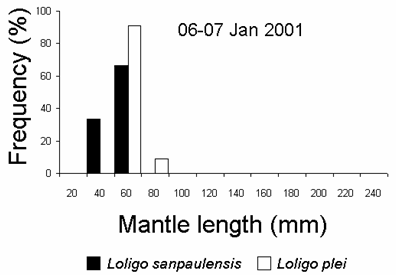
Figure 3. Size distribution of loliginid squid caught with light attraction and dipnets in 06-07 January 2001 (12 L. sanpaulensis and 11 L. plei, respectively).
In contrast with the two Loligo species, Lolliguncula brevis were often positioned in the boundary of the zooplankton and small pelagic fish schools (Fig. 1A), and exhibited a more ‘passive’ behavior, remaining within the lighted area.
On some occasions, theL. brevis assumed a distinctive flamboyant posture (Hanlon and Messenger, 1996), with all the arms held upward and the mantle pointed toward the water surface, in a more or less 45° angle, resembling the letter J (Fig. 1D). The function of this body posture is thought to be camouflage, allowing the squid to thwart predators by mimicking floating debris, such as drifting seaweed pieces (Hanlon and Messenger, 1996). This hypothesis was supported in this study because there were many predators (L. plei, L. sanpaulensis, Trichiurus lepturus) swimming around when the J posture was recorded. A similar behaviour were also recorded by Hanlon et al. (1979) for Sepioteuthis sepioidea, Abralia verany and small Ommastrephes sp. attracted by night lights at St. Croix Island, U.S. Virgin Islands, but in that case no predators were reported.
A second and very unusual behavior displayed byL. brevis was mimicry of small pelagic fish. Squid were observed mimicking the form, color and the swimming pattern of the schools ofAnchoa spp. attracted by the night lights (Fig. 1E). L. brevis is known to be associated with anchovies in Florida estuaries, where they prey upon these fishes (Ogburn-Matthews and Allen, 1993). A similar relationship could take place in coastal bights and bays off southern Brazil, with squid mimicking fish in order to hunt in disguise within the school. This is partially supported by the presence of fish remains in the few stomach contents examined (Martins, 2002). This unusual hunting technique was previously only known to be performed byS. sepioidea at Little Cayman Island, where that species mimic the shape, color pattern and swimming behavior of a local parrotfish species in order to grasp small fish (Hanlon and Messenger, 1996).
Cutlassfish (Trichiurus lepturus) behaviour
Behavioural interactions between the cutlassfish and squid were only recorded in the 1999 season. Cutlassfish were usually recorded ambushing squid and small fish that crossed the lighted area near the surface. They remained static, in a nearly vertical position, staying in the edges of the shaded area where they were relatively inconspicuous (Fig. 1A). The attacks were sudden and consisted of the fish swimming rapidly toward the prey with the mouth widely opened, biting and swallowing the squid when reached. Sometimes lunge resulted in the cutlassfish jumping out of water by up to nearly one meter. Such a hunting behavior was previously described by Martins (1992) in the ‘Porto Belo’ Bay, slight to the north of our study site. This is in line with the characteristic ambushing predatory behavior of the Family Trichiuridae (Nakamura and Parin, 1993).
In the 2000 and 2001 seasons cutlassfish caused extreme avoidance by squid, as they often disappeared completely when the fish arrived and scattered nearby. In the absence of squid, T. lepturus used the same hunting techniques described above on the small pelagic fish schools (anchovies and/or sardines) aggregated under the lights. When fish were not present, they readily attacked the squid jigs, which they often swallowed.
Small fish behaviour
Small clupeiform fish were often attracted and aggregated under the night lights. However, the swimming behaviour of the schools was quite distinctive between species. Whereas sardines (Sardinella brasiliensis, Harengula clupeola) schools were dense and swam in circles, schools of anchovies (Anchoa spp.) were somewhat ‘loose’ when compared with the sardines, and the swimming pattern was often erratic and chaotic, although the schools remained relatively packed. Sardines were also faster than anchovies, since they were difficult to capture with the dipnets (Table 1). The schooling beneath light is a typical reaction of clupeoid fish, and it is thought to be related to fish be initially attracted to the night light (positive phototaxis) and then becoming polarized by visual orientation to other individuals within the illuminated zone, and may be ‘locked’ into the schooling formation in response to this visual stimulus (Woodhead, 1966).
Among the other fish species recorded, the behaviour of the mullets (Mugil sp.) were the most remarkable, since they frequently remained immobile and with the body strongly curled, remembering a comma mark, which made them easy to catch (Table 1). The remaining fish species attracted by the lights swam erratically, getting in and out of the lighted area, with no apparent particular swimming pattern or schooling behavior. Nonetheless, Woodhead (1966) points out that species-specific behavior of fish towards artificial lights is very variable, and may also change in response to physiological and environmental factors.
Conclusions and future directions
The present note provides the first study of this kind in Brazil, providing a description of predator–prey interactions under fishing lights. The use of light attraction and dipnet also allowed the sampling of very small squids, not caught by the artisanal gears. It also provided qualitative ‘snapshots’ on the diversity of juvenile and post larvae fish and several invertebrate taxa, such as epitocal polychaetes, normally not collected by traditional sampling devices, such as conical plankton nets. Future surveys using light attraction and videotaping could be designed in order to extend squid behavioural studies in coastal waters off southern Brazil. Special attention must be paid to adequately quantify observations made at night in future studies.
References
Cohen, A. C. (1976). The systematics and distribution of Loligo (Cephalopoda: Myopsida) in the western Atlantic, with description of two new species. Malacologia, 15, 299-367. [ Links ]
Collins, M. A., & Pierce, G. J. (1996). Size selectivity in the diet of Loligo forbesi (Cephalopoda: Loliginidae). Journal of the Marine Biology Association of the United Kingdom, 76, 1081-090. [ Links ]
Costa, P. A. S., & Haimovici, M. (1990). A pesca de polvos e lulas no litoral do Rio de Janeiro. Ciência e Cultura, 42 (12), 1124-1130. [ Links ] Foyle, T. P., & O’Dor, R. K. (1988). Predatory strategies of squid (Illex illecebrosus) attacking small and large fish. Marine Behavior and Physiology, 13, 155-168.
Hanlon, R. T., Hixon, R. F., & Hulet, W. H. (1983). Survival, growth, and behavior of the loliginid squid, Loligo plei, Loligo pealei and Lolliguncula brevis (Mollusca: Cephalopoda) in closed sea water systems. Biological Bulletin, 165, 637-685. [ Links ]
Hanlon, R. T., Hixon, R. F., Forsythe, J. W., & Hendrix Jr., J. P. (1979). Cephalopods attracted to experimental night lights during a saturation dive at St. Croix, U.S. Virgin Islands. Bulletin of the American Malacological Union, 53-58. [ Links ]
Hanlon, R. T., & Messenger, J. B. (1996). Cephalopod Behaviour. Cambridge: Cambridge University Press. [ Links ]
Martins, A. S. (1992). Bioecologia do peixe-espada Trichiurus lepturus Linnaeus, 1758, no sul do Brasil. Dissertação de Mestrado, Fundação Universidade do Rio Grande, Rio Grande, RS. [ Links ]
Martins, R. S. (2002). Loliginídeos na Ilha de Santa Catarina: características e relações ecológicas, com ênfase em Loligo plei (Cephalopoda: Teuthida: Myopsina). Dissertação de Mestrado, Universidade Federal do Paraná, Curitiba, PR. [ Links ]
Martins, R. S., Perez, J. A. A., & Schettini, C. A. F. (in press). The squid Loligo plei around Santa Catarina Island, southern Brazil: ecology and interactions with the coastal oceanographic environment. Journal of Coastal Research, 39, 1285-1290. [ Links ]
Moltschaniwskyj, N. A., & Doherty, P. J. (1995). Cross-shelf distribution patterns of tropical juvenile cephalopods sampled with light-traps. Marine and Freshwater Research, 46, 707-714. [ Links ]
Moynihan, M., & Rodaniche, A. F. (1982). The behavior and natural history of the Caribbean reef squid Sepioteuthis sepioidea, with a consideration of social, signal and defensive patterns for difficult and dangerous environments.Advances in Ethology, 25, 1-151. [ Links ]
Nakamura, I., & Parin, N. V. (1993). FAO species catalogue: Vol. 15. Snake mackerels and cutlassfishes of the world (families Gempylidae and Trichiuridae). An annotated and illustrated catalogue of the snake mackerels, snoeks, escolars, gemfishes, sackfishes, domine, oilfish, cutlassfishes, scabbardfishes, airtails, and frostfishes known to date. FAO Fisheries Synopsis, 125(15), 1-136. [ Links ]
Ogburn-Matthews, M. V., & Allen, M. (1993). Interactions among dominant estuarine nekton species. Estuaries, 16(4), 840-850. [ Links ]
Perez, J. A. A., Martins, R. S., & Buratto, J. R. (1999). Estrutura e dinâmica da pesca artesanal de lulas (Mollusca: Cephalopoda) em Santa Catarina (Vol. 2, pp. 954-967). Em Anais do XII Congresso Brasileiro de Engenharia de Pesca.Olinda: Sociedade Brasileira de Engenharia de Pesca. [ Links ]
Rathjen, W. F., & Voss, G. L. (1987). The cephalopod fisheries: a review. In P. R. Boyle (Ed.), Cephalopod life cycles. Vol. 2: Comparative reviews (pp. 253-275). London: Academic Press. [ Links ]
Thorrold, S. R. (1992). Evaluating the performance of light-traps for sampling small fish and squid in open waters of the central Great Barrier Reef Lagoon. Marine Ecology Progress Series, 89, 277-285. [ Links ]
Woodhead, P. M. J. (1966). The behaviour of fish in relation to light in the sea. Oceanography and Marine Biology Annual Review, 4, 337-403. [ Links ]
 Correspondence to
Correspondence to
José Angel Alvarez Perez
Centro de Ciências Tecnológicas da Terra e do Mar (CTTMar),
Universidade do Vale do Itajaí (UNIVALI),
Cx. Postal 360, Itajaí, SC, CEP 88302-202, Brazil.
E-mail: ocersm@lycos.com
Received February 2, 2006
Revision received May 24, 2006
Accepted August 24, 2006














