Serviços Personalizados
Journal
artigo
Indicadores
Compartilhar
Revista de Etologia
versão impressa ISSN 1517-2805versão On-line ISSN 2175-3636
Rev. etol. v.8 n.2 São Paulo dez. 2006
ARTIGOS
Behavioral categories of hyacinth macaws (Anodorhynchus hyacinthinus) during the reproductive period, at South Pantanal, Brazil*
Larissa SchneiderI; Antonio Luis SerbenaII; Neiva Maria Robaldo GuedesIII
I Instituto Nacional de Pesquisas da Amazônia,Coleções Zoológicas, AM
II Universidade Federal do Paraná, PR
III Instituto Arara Azul, Campo Grande, MS
RESUMO
A Arara Azul (Anodorhynchus hyacinthinus), uma espécie abundante no início do século XIX, no Brasil, encontra- se ameaçada de extinção em toda sua área de ocorrência. A fim de contribuir ao conhecimento da espécie, realizamos observações de campo de cinco pares destas aves e descrevemos 36 comportamentos, nas categorias de manutenção, locomoção, alimentação, reprodução, interação social e vigilância. As semelhanças e diferenças em relação aos comportamentos já descritos para outras espécies de psitacídeos são discutidas.
Palavras-chave: Arara azul, Anodorhynchus, Psitacidae.
ABSTRACT
The hyacinth macaw (Anodorhynchus hyacinthinus), abundant in Brazil at the beginning of the 19th century, is now endangered throughout its range. As a contribution to the knowledge of this species, we give a description of its species-typical behaviors, categorized as maintenance, locomotion, feeding, reproduction, social and vigilance categories, based on field observations with five pairs of birds in South Pantanal, Brazil. Similarities and differences with the repertoires of other psitacid species are discussed.
Keywords: Hyacinth macaw, Anodorhynchus, Psitacidae.
Anodorhynchus hyacinthinus, abundant at the beginning of the 19th century, is now considered an endangered species in its whole occupancy area (Guedes, 1995; Munn, Thonsen, & Yamashita, 1997). As the biggest species of the Psittacidae family it may reach one meter length (Sick, 1997) and weigh 1,3 kg, It occupy only woodland and savanna habitats (Brandt & Machado, 1990; Forshaw, 1989; Sick, Gonzaga, & Teixeira, 1987; Yamashita, 1987, 1992). In nature, Hyacinth macaws live in pairs or in flocks and nest in cavities of tree trunks (Sick, 1997). They are highly social birds, living in pairs, families or groups, in relatively sedentary populations that can make short daily migrations for foraging and/or reproduction. The hatchlings stay in the nest for an average of 107 days. After leaving the nest, they are still fed by their parents for about 6 months, when they start trying to break nuts by themselves. Most chicks remain in the company of their parents for approximately 18 months, after which they usually join other groups of young macaws (Guedes, 2002).
The reproductive period starts in July/August and birds at this time select a cavity to nest (Guedes, 1995). Eggs are incubated by the female who stays in the inside of the cavity most of the time, leaving to the male the task of feeding her. The incubation period varies between twenty-eight and thirty days and the nestling are kept inside the nest for about a hundred and seven days (Guedes, 1995).
There is information about the biology of A. hyacinthinus (Guedes, 1995; Pinho, 1998; Yamashita, 1993, 1997) but behavioral studies are scarce and the description of basic categories of behavior, in the form of an ethogram, is still needed. Ethograms are important starting points for ethological research and for a full understanding of the biology and ecology of animals (Lehner, 1996). Our objective here is to establish a catalogue of A. hyacinthinuss behavioral categories as displayed by pairs during the reproductive period, as a contribution to future studies on breeding and conservation of the hyacinth macaw.
Methods
Study area. The study was conducted in the Refúgio Ecológico Caiman (19º57S, 56º17W). This ranch is located in the Miranda sub-region of South Pantanal (Adámoli, 1982), state of Mato Grosso do Sul, Brazil. The Pantanal wetland is a large floodplain located in the upper Paraguay River basin. Seasonal inundation of the floodplain is the main ecological factor in the Pantanal Ecosystem (Adámoli, 1982). Its vegetation consists of a mosaic of several forested and open habitats that vary in topography and flooding regime (Prance & Schaller, 1982). The rainy season occurs between November and April with a dry season between May and October (Cadavid Garcia, 1984).
Behavioral observations. The data were collected from June 2001 to January 2002 during day time (from 06:00 to 18:00hr) using an ad libitum method (Martin & Bateson, 1991) by identifying the conducts and grouping into categories. We followed the names given in the literature of behavioral items already described. New items were named according to the context in which they were observed. Observers stayed at about 100 meters from the nests, under a camouflaged tent, below a tree, and used a 7 x 50 binocular and an eyeglass 60mm, in order to be as unobtrusive as possible. A minimum period of 2 days per month was scheduled to allow a better adaptation of pairs to the observers.
Identification of individual A. hyacinthinus, a difficult task, was done through discrimination of little marks on the beak of birds. To identify the females, we used as a cue the feathers which get curly because of the long time spent inside the nest cavity. We also differentiated male and female through behavior, as described for Ara rubrigenys (Lanning, 1991). Pictures were taken of the main categories and were the base for sketches.
Results
280 observation hours were spent in the field. 35 behavioral items were described, in six general categories: maintenance, locomotion, feeding, reproduction, social and vigilance.
Maintenance
Preening. The macaws basically use their beak and tongue to groom, twisting their head and body towards the area to be groomed. There are differences in the behavior due to the size of the feathers. Where the feathers are short, the macaws usually just nibble them. Long feathers are separated one by one. The upper mandible (as a hook) separates the feather which is supported by the lower mandible, slipping this feather between beak and tongue. During this performance, macaws often pass their tongue and beak through the uropygial gland to lubricate the feathers. Grooming starts at the upper part of the feather shaft, and the feather is slipped throughout between beak and tongue.
The tails feathers are pulled it through the head, in such a way that make their head get back to the position directed to the front. The birds very frequently bristle the feathers to help the grooming.
Slipping of feathers is very common at dawn and sunset. During the dry season, in the hottest time of the day, the macaws stay on a bough and perform heat loss behavior, Behavioral categories of hyacinth macaws dropping the preening frequency and increasing it at dawn and sunset. They usually switch between resting and alert behavior when performing this behavior.
Allopreening. Analogous to preening in performance, it may help macaws to get some body part preened more efficiently.
Mutual allopreening. The head of one bird reaches the belly of the other and vice-versa. They usually nibble the cloaca of each other.
Internal Beak cleaning. One of the legs is raised towards the beak, and the birds bend their head down softly until the toes reach the internal part of the beak and are passed through the cavities. Sometimes, they may even use their legs to open the beak wider and move the tongue easier. Como assim ? Refazer a descrição.
Outward beak cleaning. Macaws, when landed, clean the outside surface of their beak either by rubbing it against branches, twisting down the head or by introducing the beak into holes of branches and scratching it against the rim.
Bathing. Macaws look for small water holes around the nest boundaries. Birds bend first and plunge their body totally or partially in the water. They then stand up and shake.
Stretching. Macaws standing on a branch or on the nest edge may raise one of their legs and stretch it, to the opposite side of the other leg (about 45º). They may bend their heads a little bit and they stretch their feet slightly and also stretch wings and tail feathers opening them as a fan.
Pinching. The head is bent to reach a branch, with a slight raising of the tail. Macaws then pinch the branch with abrupt movements, using the upper mandible. This is sometimes performed upside down. Pinching may sharpen the beak and may be used to make the nest cavity.
Resting. Macaws keep motionless, on a branch, tail down and head up This behavior was always recorded in alternation with snoozing and vigilance behaviors.
Snoozing. Macaws at rest may close their eyes and snooze quickly.
Yawning. During yawning, the beak is opened, the mandible is raised. The yellow external part increases about 50% of its size,
Panting. This behavior is performed after long distance flights and also under strong sunshine. Macaws land on a branch or at the nest edge and open the beak, moving the tongue up and down, which may help then to loose heat. Feathers are brought close to the body, avoiding thus air accumulation between the feathers and increased body temperature. Wings may main at some distance from the body.
Scratching. Macaws reach the area to be scratched with the leg, twisting often their body. They may also rub their head on the branch.
Sharpening the beak. Macaws sometimes manipulate small rocks, pieces of tree bark or tiles between the upper and lower mandibles. They may also sharpen their beaks by slipping a round object between the beak together with a stem (Figure 1). They hold the stem into the beak by using two fingers, one on the right side, the other one on the other side of the beak. Tongue and maxilla move in such a way that the object spins on the beaks blade.
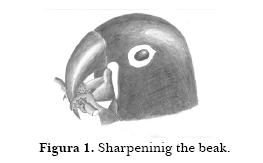
Shaking feathers. They bristle the feathers (especially the ones on the neck) and shake the whole body twice or three times straight away, during one or two seconds. Sometimes they slightly open their wings during this behavior.
Locomotion
Flying: The macaws move to the point where they are going to take off and bend their head and body down, head slightly lower than Figura 1. Sharpeninig the beak. the body and tail at the same level as the body line. The birds then thrust with the feet and give one strong horizontal wing flap. Once started, the birds perform constant flying wing flaps, keeping the head at the same level as or slightly lower than the body.
Landing. When landing, the macaws begin increasing the wings angle and then raise their heads and flap their wings lower and horizontally, tail feathers open, as if to help the breaking process.
Flying over: Adults vigorously defend the nest by squawking loudly and performing flying over behavior (Guedes, 1995). Instead of flying straight, as in normal flying, the birds fly around the nest, swooping against the intruder.
Walking. This behavior is similar to walking in other psittacidae (Amazona pretrei, Prestes, 2000; A. hyacinthinus, Yamashita, 1987, 1992). The macaws alternate paws, inclining the body to the opposite side of the foot that is ahead. Walking on the ground, the macaws leave their feet slightly separated, dragging their tail. The macaws can move laterally, faster than in regular walk (as do Amazona pretrei, Prestes, 2000).
Jumping. The macaws thrust with their feet and perform a fast wing flap to jump higher, from one branch to another one.
Climbing: When macaws are entering or leaving the nest, they climb the nest edge using beak and feet. They fix the beak on the edge and move their bodies up, then fix the feet and move the beaks up, until getting into the nest.
Feeding behavior
Mainly arboreal seed predators (Janzen, 1971; Yamashita, 1992), hyacinth macaws usually forage in the canopy of tall trees. In the study period, they were seen feeding on two species of palm tree, the bocaiúva (Acrocomia aculeata - Palmae) and the acuri (Scheelea phalerata- Palmae).
Plucking fruits. On some occasions, the macaws were seen plucking almost ripe (yellow) fruits from the acuri palm trees. Using the beak, they took the fruits from the bunch and left them fall on the ground. About one month later, the macaws went back to the same palm tree and ate the endosperm of those fruits, which were perforated and had eventually the larva of a non-identified insect inside. Macaws could then forage on such insects.
Fruit manipulation: When flying to forage, either the macaws leave together; or it is the male which takes off singly. They land on the top of palm trees and go to the bunch of frutis, at the lower part of the canopy. They climb, with legs and beak, to the top of the bunch and then pick up the fruits with the beak. As described by Borsari and Ottoni (2005), macaws position the fruit beneath their upper mandible, always holding the fruit with one foot, and start grooving it by pushing with the lower mandible. In nature, the macaws were seen fixing the right foot on the bunch, while the left held the fruit in the beak (or vice-versa). They held the fruit between front and back toes. Then, once set in this position, they cracked open the fruit with the beak.
Macaws remain eating at the same tree or flight at other places, farm fences, animal pens or other trees. They were sometimes seen manipulating the fruits using, as tools, leaves from small acuri or bocaiúva branches. Such leaves have a rough texture which prevents the fruit from slipping from the macaws beak. Macaws take off the leaf from the tree and wrap the fruit up using the leaf. The leaf helps the macaw to open the mesocarp, preventing the dropping out of the fruit. Fruit manipulation performance differs according to the plant species, but some behavioral items are similar across episodes:
1. The macaw peels up the exocarp, fixing the fruit with the beak and pulling in the fibred peel from bottom to top (Figure 2A). 2. The macaw detaches the mesocarp manipulating it from bottom to top (Figure 2B). The longitudinal direction of breaking the monocarp fibers is appropriate to the structure of the fruit which cannot be treated transversally as the macaws beak is smooth and not serrated. 3. The macaw presses the fruit to cut it transversally into two parts (Figure 2C). The two parts are both held but are manipulated one at a time. 4. The macaw takes out the endosperm by spinning the seed using the fingers. (Figure 2D). Rarely do they eat the mesocarp, and never the exocarp, which is usually dropped a under the tree.
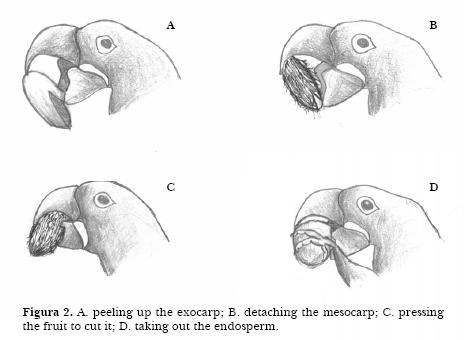
When eating acuri, the macaws follow all parts of the sequence. Macaws were sometimes, but rarely, seen using a leaf to wrap up the fruit. They were also observed slicing off a piece of wood from the perch, which was then positioned immediately beneath the birds upper mandible, with the aid of the tongue. Borsari and Ottoni (2005) described the same behavior for Hyacinth macaws in captivity eating indaia nuts. The animal held the nut with its foot in contact with the fixed piece of wood. Using its lower mandible, the macaw pushed the nut against the tool, again grooving it with its lower mandible. The bird repositioned these items several times with its tongue, beak and/or foot.
With unripe acuri, the endosperm of which is liquid, the macaws do not perform the items listed above. They cut the fruit in half, and drink the endosperm. For ripe bocaiúva, macaws use basically the same movements, but do not perform the taking out of the exocarp: the bocaiuva has a thin and breakable exocarp that is taken out together with the mesocarp. When eating ripe bocaiuva, the macaws very often use tools to wrap up the fruit and make the manipulation easier. We never, in this study, observe the macaws eating unripe bocaiuva. Guedes (1995), however, noticed that macaws drank the liquid endosperm of bocaiuva as well of unripe acuri.
Regurgitating: This behavior occurs during food exchange between male and female. Moving his head forward, up and down, the male fixes the beak to the females. Food goes from male to the female, through a synchronized movement of body and tongue. During the nesting period, the macaws go both to a palm tree to forage and the male regurgitates the food to the female. The female does not feed the male. When, later, the female incubates the eggs, the male protects the nest and feeds both. During this period, the female is totally dependent on food brought by the male. Courtship feeding behavior, very common during nesting period, decreases gradually in frequency later.
Thrashing in cattle excrement: domestic cattle, especially the more generalist Brahman races, readily ingest palm fruits (Yamashita, 1997), ruminate and regurgitate them and usually eliminates them in their excrements (Guedes, 1995). Such processes were not here observed once they occur more commonly at night (Yamashita, 1997). It was however possible to find bare ground patches littered with manure with regurgitated seeds. In such cases, we observed the macaws landing around the patches and forage for seeds which were cut and the endosperm of which was eaten.
Drinking: The macaws bend down, and raise their head, putting their tongue up.
Defecating: Macaws land on the branch and remain motionless while defecating, during a few seconds.
Reproductive behavior
Copulating: After mutual allopreening, the birds place themselves back to back, head down and tail up in a way that the cloacas come together. Beaks are open and tongues move up and down. The birds use their beaks to keep their balance on the branch where they are landed. Sometimes they grasp their partner with the feet (the left macaw fixes with the left foot and vice-versa). During copulation, macaws produce a specific vocalization which cadences until the whole process ends (it starts low, increases in rhythm and ends in sharp vocalizations, here called co-co vocalization). It lasts around three minutes and can be performed several times a day. It is usually produced when the macaws are getting the nest cavities ready and is less common during incubation.
Copulation categories are: 1. Macaws ruffle their feathers and start allopreening the head (Figure 3A). 2. The head of one individual is directed to the others cloaca and allopreening of the cloaca begins (Figure 3B). 3. Animals adopt the back to back copulation position, directing their heads forward and down, raising their tail up (Figure 3C); they start the co-co vocalization touching the cloaca of each other. 4. They next touch the surface with the head. while one of their feet placed on the substrate and the other either on the substrate also or on the partners body (Figure 3D). Vocalization reaches then its highest intensity, tongues move up and down and the animals consummate copulation. 5. Macaws decrease and end vocalization, separate the cloacas and put down their tails.
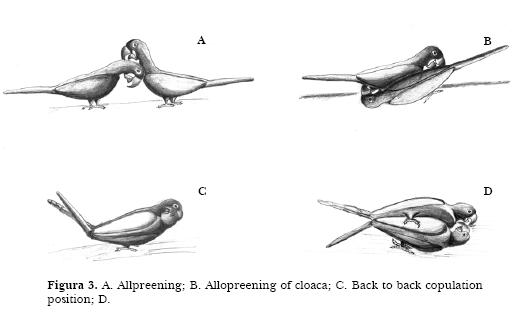
Female may reject males attempt to copulate. The male rises the tail trying to put cloacas in contact but the female lowers her tail, preventing contact of cloacas, till the male gives up. The female may display then starts a short allopreening bout.
Social behavior
From the moment they select a site for their nest to the moment nestling fly, male and female macaws remain most of the time together, separating only when the male flies away to search food. In occasions in which the territory is invaded by another species, as the vulture Coragyps atratus, the male performs intense interspecific agonistic behaviors. Competition is not restricted to the site of the nest, it may expand to the entire area that the couple dominates. The female is rarely involved in fighting. As soon as the intruder enters the area and is detected, the male quickly flies and attempts repelling it in several ways.
Intraspecific defense (intimidation). When an intruder macaw invades the feeding area, the couple first produces different kinds of loud squawking, to which the intruder responds with loud vocalizations. Loudness of the vocalization is an important feature in dominance contests. After spending a long time vocalizing, the nest pair starts intimidation, during which male and female adopt a position similar to the copulation posture and start vocalizing as in copulatory episodes. They however do not unite cloacas. During our study, intimidating behavior always preceded agonistic interactions in the nesting and feeding areas.
Inter and intraspecific defense (substitution attack). In case the nest is invaded, the resident macaw jumps towards invaders, which may be macaws or other animals, such as black vultures. The resident male (and sometines the female) may beak the intruder. Behaviors displayed are: 1. The resident individual jumps towards the invader, causing it to fly away or to perch in another branch (Figure 4 A). The resident male then continues pursuing the intruder, flying in its direction. 2. The resident macaw flies towards the invader, but this time using the beak to hurt the invader until this is repelled (Figure 4B). The female is sometimes enrolled in this defensive display.
Individual Play. The birds perform somersaults on the branch, sometimes staying upside-down, fixed only by the feet or by the beak (body is then loose and the animals beat quickly their wings). This behavior is very often performed on the palm trees when macaws jump near bunches: they may disperse the fruits collected through the air.
Social Play. Individuals get close and peck at each other, when perched on a branch. They sometimes produce a typical, short lasting and low intensity vocalization.
Vigilance
Vigilance activity is intense during the reproductive period and has probably as its function to patrol the territorial area and to guarantee the nestling protection. Other species display similar vigilance behaviors (Vanellus chilensis, Costa, 1994; Calyptorhynchus baundinii, Saunders, 1974).
Watching behavior. This behavior may be performed by a single animal or by the pair. Inside the nest, or near it, individual macaws keep inspecting the area by twisting their heads and eventually their body in several directions. Social watching is similar but may be marked by a lower rate of twistings. It was frequently recorded when macaws foraged.
Checking the nest. The male checks the nest before the female enters by putting his head inside it (tail up, head down), during a period which may last for some minutes. This behavior is performed after long absence or after an invasion.
Discussion
Most of the categories we described for A. hyacinthinus do not differ markedly from those of other psitacidae species, such as the redspectacled parrot (Amazona pretrei, Prestes, 2000). There are however differences in performance between these two species and new categories for A, hyacinthinus. Locomotion is similar in both species, but A. hyacinthinus was watched performing, besides locomotion proper, climbing and flying over, categories not described for A. pretrei.
Maintenance behaviors were described for Amazona pretrei (Prestes, 2000), Triclaria malachitacea (Bencke, 1998) and Ramphanstos toco (Mikich, 1991). We observed some fifteen maintenance behaviors similar to those seen in other Psitacidae species. A. hyacinthinus has, however, a peculiar way to manipulate objects to sharpen the beak. Macaws showed a great ability to manipulate little stones, not for the moment described in other species.
Panting behavior is performed by A. hyacinthinus as it is performed in other species. This behavior has an important function during the reproductive period, which happens at the beginning of the dry season. It may serve to cope with high temperatures and with intense flying effort spent during the protection of the nest area. The stretch behavior seen during the reproductive period may be adaptive for the female, forced to remain motionless for long periods, in the nest. Allopreening and mutual allopreening behaviors may probably represent a kind of grooming exchange (described in Calyptorhynchus baundinii, Saunders, 1974), favoring the pair bonding. In A. hyacinthinuss case, these behaviors may play a specially important tie-reinforcing role for the reproductive pair, since both male and female quite frequently forage among conspecifics.
In the feeding category, some of our descriptions, such as thrashing cattle excrement and the handling of fruit, are new. The regurgitating behavior watched in this study does not differ from others already described. A. hyacinthinus are high specialized to eat two palm fruit species bocaiúva (Acrocomia aculeata) and acuri (Scheelea phalerata), their way to manipulate the fruits using leaves is different from the way observed in other species. The acuri slower and circumstantial manipulation is compensating from the point of view of the energy quantity that the macaws can get (Carciofi, 2002).
Macaws in nature, unlike those studied in captivity (Borsari & Ottoni, 2005), were watched using tools only during the fruit´s mesocarp removal, not to manipulate mesocarpfree nuts. Borsari and Ottoni (2005) suggest that a piece of wood may prevent the nut from rotating inside the birds beak, serving as a wedge and facilitating the grooving of the nut. Alternative or complementary functions might be: reducing the impact of cracking, preventing the nut from slipping, and/or providing mechanical aid in its positioning and use of force.
Thrashing cattle excrement is not not included in previous ethograms of macaws behavior. This behavior originate from the specialization of A. hyacinthinus on palm seeds as staple food as well as from the commensal relationship between macaws and cattle (already described by Yamashita, 1997). Advantages of obtaining food in this manner are economy of effort: fruits does not have to be collected on trees and, in excrements, they come without exocarp and mesocarp.
Foraging on fallen fruits may represent a way of obtaining protein from insect ingestion. Fallen fruits are perforated by unidentified larvae. Carciofi (2002), in a study of Hyacinth macaw feeding, found two beetles species from the Bruchidae family, Pachymerus nucleorum and Pachymerus cardo, in the fruits the macaws ate and suggested that searching for insects might be related to protein demand of the birds.
Reproduction is the behavior in which we found most differences relatively to prior studies. Reproductive behavior is difficult to observe under field conditions and has been frequently studied in captive animals. There is a lack of comprehensive ethograms such as Saunders (1974) in the case of Calyptorhynchus baundinii.
During the reproductive period of A. hiacinthinus, there is a clear division of tasks between male and female: males spend most of their time interacting with invaders to protect the nest area, while females provide care for nestling. Task division task, the constant female attendance by males, the female permanence inside the nest, and the successive copulations may represent a selective consequence of sperm competition. Such behaviors decrease the chances of females extra-pair copulation (Rodrigues, 1998). What may be thought as cooperative behavior between mates, in fact may represent a conflict of interest based on the differences of reproduction investment between sexes (Trivers, 1972). There are records of purely monogamous birds, such as the albatross (Phoebastria irrorata), with as much as 25% of offspring which result from extra-pair fertilization (Huyvaert, Anderson, Jones, Duan, & Parker, 2000). Fujioka and Yamagishi (1981) noted in the cattle egret (Bubulcus ibis) that the highest frequency of extra-pair copulation and defense occurred during the nest-building and egg-laying period (fertile period). Future research is needed to assess the occurrence or absence, in the case of Hyacinth macaws, of extra-pair copulation.
The description of substitution attack is another contribution of the present study. Macaws perform this category adjusting it to the invader species as well as to the intensity of the agonistic event. It should be studied further in order to clarify the differences and relationship between copulation and intimidating behaviors, especially as concerns the vocalizations produced.
References
Adámoli, J. (1982). O Pantanal e suas relações fitogeográficas com os Cerrados. Discussão sobre o conceito de Complexo do Pantanal. Em Livro de Resumos. XXXII Congresso Nacional de Botânica (pp. 109-119). Teresina, PI: Sociedade Brasileira de Botânica. [ Links ]
Bencke, G. A. (1998). Ecologia do Sabiá-cica [Triclaria malachitacea (Spix, 1824)] em fragmentos florestais remanescentes do estado do Rio Grande do Sul: ocupação do ambiente e alimentação. Dissertação de mestrado, Universidade Estadual Paulista, Rio Claro, SP. [ Links ]
Borsari, A., & Ottoni, E. B. (2005). Preliminary observations of tool use in captive hyacinth macaws (Anodorhynchus hyacinthinus). Animal Cognition, 8, 48–52. [ Links ]
Brandt, A., & Machado, R. B. (1990). Área de alimentação e comportamento alimentar de Anodorhynchus leari. Ararajuba, 1, 57-63. [ Links ]
Cadavid Garcia, E. A. (1984). O clima no Pantanal Mato-grossense. Circular Técnica EMBRAPAUEPAE, 14. [ Links ]
Carciofi, A. C. (2002). Estudos sobre nutrição de psitacídeos em vida livre: o exemplo da araraazul (Anodorhynchus hyacinthinus). Em M. Galetti & M. A. Pizo (Eds.), Ecologia e conservação de psitacídeos no Brasil (p. 236). Belo Horizonte: Melopsittacus Publicações Científicas. [ Links ]
Costa, L. C. M. (1994). Manobras de distração de Vanellus chilensis (Wagler, 1827) (Charadriiformes, Charadriidae) em Curitiba, Paraná, Brasil. Estudos de Biologia, 3(36), falta página. [ Links ]
Forshaw, J. M. (1989). Parrots of the world (3rd. ed.). London: Blanford Press. [ Links ]
Fujioka, M., & Yamagishi, S. (1981). Extramarital and pair copulations in the cattle egret. The Auk, 98, 134-144. [ Links ]
Guedes, N. M. R. (1995). Alguns aspectos sobre o comportamento reprodutivo da arara-azul Anodorhynchus hyacinthinus e a necessidade de manejo para a conservação da espécie. Em Anais. XIII Encontro Anual de Etologia (pp. 274-292). Pirassununga, SP: Sociedade Brasileira de Etologia. [ Links ]
Guedes N. M. R. (2002). The hyacinth macaw project in the South Pantanal, Brazil. In Annals. Vth International Parrot Convention (pp. 163-174). Tenerife, Spain. [ Links ]
Huyvaert, K. P., Anderson, D. J., Jones, T. C., Duan, W., & Parker, P. G. (2000). Extra-pair paternity in waved albatrosses. Molecular Ecology, 9, 1415- 1419. [ Links ]
Janzen, D. H. (1971). Seed predation by animals. Annual Review of Ecology and Systematics, 2, 465-492. [ Links ]
Lanning, D. V. (1991). Distribuition and breeding biology of the red-fronted macaw. Wilson Bulletin, 103(3), 357-365. [ Links ]
Lehner, P. N. (1996). Handbook of ethological methods. Cambridge: Cambridge University Press. [ Links ]
Martin, P., & Bateson, P. (1991). La medición del comportamiento. Madrid: Alianza. [ Links ]
Mikich, S. B. (1991). Etograma de Ramphastos toco em cativeiro (Piciformes: Ramphastidae). Ararajuba, 2, 3-17. [ Links ]
Munn, C. A., Thonsen, J. B., & Yamachita, C. (1997). Estudio y situacion de Ara Jacinto (Anodorhynchus hyacinthinus) en Brasil, Bolívia y Paraguay. Preparado para la Secretaria de la Convención sobre el Comercio Internacional de las Especies Amenazadas de Flora Y Fauna Silvestres (CITES). [ Links ]
Pinho, J. B. (1998). Aspectos comportamentais da Arara- Azul (Anodorhynchus hyacinthinus) na localidade de Pirizal, Município de Nossa Senhora do Livramento – Pantanal de Poconé. Dissertação de mestrado, Universidade Federal do Mato Grosso, Cuiabá, MT. [ Links ]
Prance, G. T., & Schaller, G. B. (1982). Preliminary study of some vegetation types of Pantanal, Mato Grosso, Brazil. Brittonia, 34(2), 228-251. [ Links ]
Prestes, N. P. (2000). Descrição e análise quantitativa do etograma de Amazona pretrei em Cativeiro. Ararajuba, 8(1), 25-42. [ Links ]
Rodrigues, M. (1998). The role of the sperm competition in the breeding behavior of birds: Testing the predictions. Ciência e Cultura, 50(6), 437-445. [ Links ]
Saunders, D. A. (1974). The function of displays in the breeding of the white-tailed black cockatoo. MEU, 74, 43-46. [ Links ]
Sick, H. (1997). Ornitologia brasileira. Rio de Janeiro: Nova Fronteira. [ Links ]
Sick, H., Gonzaga L. P., & Teixeira, D. M. (1987). A arara azul de Lear, Anodorhynchus leari Bonaparte, 1956. Revista Brasileira de Zoologia, 3(7), 441-463. [ Links ]
Trivers, R. L. (1972). Parental investment and sexual selection. In B. G. Campbell (Ed.), Sexual selection and the descent of man 1 (pp. 36-179). Chicago: Aldine. [ Links ]
Yamashita, C. (1987). Field observations and comments on the Indigo Macaw (Anodhorhynchus leari), a highly endangered species from northeastern Brazil. Wilson Bulletin, 99, 280-282. [ Links ]
Yamashita, C. (1992). Comportamento de ararauna (Anodorhynchus hyacinthinus) Psittacidae, Aves. Anais de Etologia, 10, 158-162. [ Links ]
Yamashita, C., & Barros, Y. M. (1997). The bluethroated macaw Ara glaucodularis: Characterization of its distinctive habitats in savannahs of the Beni, Bolivia. Ararajuba, 5(2), 141-150. [ Links ]
Yamashita, C., & Valle, M. P. (1993). On linkage between Anodorhynchus macaws and palm nuts, and the extinction of the Glaucous Macaw. Bulletin of British Ornithological Club, 113(1), 53-60. [ Links ]
 Endereço para correspondência
Endereço para correspondência
Larissa Schneider
Av. André Araújo, 2936, Aleixo
69060-001, Manaus, AM, Brasil
E-mail:laribio@terra.com.br
Recebido em 29 de outubro de 2006
Aceito em 5 de outubro de 2007
* We thank M. Uetanabaro, Y. Barros, L. C. M. Costa, T. M. Sanaiotti and M. Cziulik who contributed with suggestions to this manuscript. A. Akemi, L. Guedes and C. Correa for helping the field work. Teresa Lathan and L. P. Schneider for helping translate the article. UNIDERP, FMB, Toyota, WWF – BRASIL, Refúgio Ecológico Caiman, Conservation International, Instituto Arara Azul and Vanzin for supporting the whole study.














