Serviços Personalizados
Journal
artigo
Indicadores
Compartilhar
Journal of Human Growth and Development
versão impressa ISSN 0104-1282
Rev. bras. crescimento desenvolv. hum. vol.20 no.3 São Paulo 2010
ORIGINAL RESEARCH PESQUISA ORIGINAL
The contribution of arterial versus venous vascular permeability in peritoneal fluid formation
Igor Ivanecky; Jaques Belik
The Hospital for Sick Children, University of Toronto, Toronto, Canada
ABSTRACT
OBJECTIVES: peritoneal fluid accumulation is a common finding in many children with abdominal disorders and its generation secondary to increased vascular permeability. The contribution of the arterial versus venous circulation to edema formation and peritoneal fluid accumulation is poorly understood. Studies conducted in vivo more than two decades ago suggested that the postcapillary venules were more important than the arterial vessels in the process of edema formation. The purpose of the present study as to evaluate the effect of changes in intravascular pressure and the inflammatory mediator bradykinin on rat mesenteric arterial and venous vascular permeability.
METHOD: mesenteric arteries (MA) and veins (MV) were mounted on glass cannulas, intravascularly filled with fluorescent dextran and incrementally pressurized above their in vivo physiological values. Vascular permeability to dextran was determined at 100, 200 and 300 % of physiological pressures.
RESULT: vascular permeability was present at all measurements for both vessels and its magnitude directly proportional to the intravascular pressure. Bradykinin (10-5 M) significantly increased permeability in the MV but not in the MA.
CONCLUSION: the abdominal fluid accumulation related to bowel inflammatory disease is more likely to be secondary to venous, as opposed to arterial vascular leakage.
Key words: capillary permeability; muscle smooth; vascular endothelium; vascular; Bradykinin; Cardiovascular System.
RESUMO
OBJETIVO: o acúmulo de líquido peritoneal é um achado comum em muitas crianças com distúrbios abdominais e sua geração é secundária ao aumento da permeabilidade vascular. A contribuição da circulação arterial versus venosa para a formação de edema e acúmulo de líquido peritoneal é mal compreendida. Estudos realizados in vivo mais de duas décadas atrás, sugeriram que a vênulas são mais importantes que os vasos arteriais no processo de formação de edema. O objetivo deste trabalho é analisar os efeitos das mudanças na pressão intravascular e a inflamação mediada pela bradicinina na permeabilidade venosa e vascular mesentérica de ratos.
MÉTODO: artérias mesentéricas (MA) e veias (MV) foram montadas em cânulas de vidro, por via intravenosa preenchida com dextran fluorescentes e incremental pressurizado acima de seus valores fisiológicos in vivo. Permeabilidade vascular para dextrana foi determinada a 100, 200 e 300% de pressões fisiológicas.
RESULTADOS: a permeabilidade vascular estava presente em todas as medidas para ambos os vasos e sua magnitude diretamente proporcional à pressão intravascular. A bradicinina (10-5 M) aumentou significativamente a permeabilidade da MV, mas não no MA.
CONCLUSÃO: o acúmulo de líquido abdominal relacionado à doença inflamatória intestinal é mais provável de ser secundária a venosa, em oposição à fuga vascular arterial.
Palavras-chave: Permeabilidade capilar; músculo liso; vascular; Eedotélio vascular; Bradicinina; sistema cardiovascular.
INTRODUCTION
The factors controlling vascular permeability in health and disease are poorly understood. Changes in vascular permeability are commonly present in certain clinical diseases and when occurring in the lung or brain lead to serious consequences related to impaired gas exchange and cerebral function, respectively.
Another manifestation of extravascular leakage is peritoneal fluid accumulation associated with bowel inflammation. In neonates and older children, this often relates to conditions such as necrotizing enterocolitis, appendicitis or other abdominal organ inflammation. Abdominal fluid accumulation occurs when fluid leaks out from the intravascular space at a faster rate than can be absorbed back into the lymph circulation, resulting in fluid accumulation. Prevention and proper treatment of peritoneal fluid accumulation requires a clear understanding of the mechanism involved in the process.
Endothelial cells regulate the passage of gases, fluid and various molecules across blood vessels by acting as selective filters. The vascular endothelium is formed by a sheet of endothelial cells tethered together by junctional proteins such as tight and adherens junctions. These cell-to-cell connections allow for the formation of inter-endothelial gaps through which substances can pass across from one side of the vessel to the other. This process is known as paracellular transport and it is one manner through which permeability is mediated.
The second mechanism involves the transcellular pathway. In it, macromolecules are transported across the endothelium via caveolae, vesicles or other intracellular organelles through pinocytosis. Though evidence for the existence of these pathways is fairly extensive, not much is known regarding their individual contributions to permeability changes in vessels.
To date, most studies have focused on the permeability of specific vascular networks in vivo, or on permeability properties of a single type of vessel. In vivo studies, in which Evans blue dye was injected into the circulation, have shown that an increase in blood pressure can increase the amount of dye that accumulates in the interstitial fluid1. This along with the presence of filtration slits in the endothelium of both arteries and veins gives rise to the hypothesis that vascular permeability is directly proportional to intravascular pressure. However, these in vivo studies did not investigate whether the increased extravasation into the tissues was due to increased permeability of veins or arteries
Limited data are available regarding a venous and arterial permeability comparison as a function of intravascular pressure. Histologically, arteries and veins have striking differences that are likely to play a role in their relative permeabilities. Arteries have overall thick walls with greater smooth muscle, elastic tissue and collagen surrounding the inner wall of endothelium. In contrast, veins have walls that are relatively thin with a thin layer of collagen fibers and connective tissue and very little to no smooth muscle present. Since a thinner vessel wall creates less of an impediment to the movement of substances across, it was hypothesized that veins will be more susceptible to an increase in permeability as a result of increased intravascular pressures when compared to arteries. Therefore the main goal of the present study was to comparatively evaluate in vitro mesenteric artery (MA) and vein (MV) vascular permeability at increasing intraluminal pressure.
In addition, we evaluated the effects of the proinflamatory neuropeptide bradykinin on the permeability of the vessels. Medeiros et al.2 and Regoli et al.3 have shown that proinflammatory responses tend to increase expression of the bradykinin â receptor in the mesenteric vein of the rat. Since increased vessel permeability accompanies inflammatory responses, these findings suggest there may be a role for increased permeability in veins caused by bradykinin. It has been largely assumed that bradykinin exerts its actions on the postcapillary microvessels4-7, but no studies have looked at the leakage in veins. We hypothesized that bradykinin increases vascular permeability in mesenteric veins but has no effect on mesenteric arterial permeability.
METHOD
Animals
Adult female Sprague-Dawley rats were used for all experiments, since technically the required measurements are not feasible in newborn or juvenile animals. The rats were housed in the Animal Care Facility at the Hospital for Sick Children and all procedures were approved by the institutional animal care committee. The animals were sacrificed using a lethal injection of sodium-pentobarbital and the mesenteric bed was extracted and placed into a cold Krebs-Henseleit solution and maintained at a pH = 7.4 ± 0.04 until vessel dissection.
Preparation of Mesenteric Segments and Pressurized Arteriograph System
A second order branch of the MA or MV was carefully dissected out and mounted on glass cannulas in an arteriograph chamber, as previously described8. The cannulas were attached to a pressure transducer and controller that allowed intravascular pressure to be set to desired values and maintained constant. The chamber in which the vessel was mounted contained a glass bottom and a camera beneath that allowed for continuous monitoring of changes in vessel diameter using the VediView Software (DMT incorporated, Denmark).
Measurement of Vascular Permeability
Using Flurescent Dextran
After mounting on the arteriograph the MA or MV were infused with 10-5 M fluorescent dextran dissolved in Krebs-Henseleit solution. The vessels were pressurized to physiological values (MA: 70 mmHg, MV: 15 mmHg) and the intraluminal pressure monitored continuously with an inline pressure transducer. Only vessels that maintained stable intraluminal pressure were studied. Next, the arteriograph bath chamber was thoroughly washed out with Krebs-Henseleit solution to remove any extravascular dextran.
Measurements of dextran leaking out into the vessel chamber as a result of vascular permeability changes were determined by collecting the arteriograph bath fluid samples (20µl) in triplicates. The collected fluid fluorescence was measured with a fluorometer set with excitation and emission wavelengths of 494nm and 521nm respectively.
Pressure Curve Experiment
Vessels were pressurized to physiological values and increased in stepwise manner to 200 % and 300 % of the initial settings. Arteriograph bath fluid samples were obtained prior to, immediately after and following 20 min post changes in intravascular pressure as outlined in Figure 1. Vessel viability was checked at the end of each experiment by measuring the vasoconstrictor response to phenylephrine (10-3 M).

Bradykinin Experiment
Vessels were slowly pressurized to their in vivo physiological values, as described above. The fluorescence measurements were taken initially and after 20 minutes without any treatment and this was used as basal values. Bradykinin was added to the vessel chamber for a final concentration of 10-5 M and the fluorescence measurements repeated after 20 min.
To further ensure that bradykinin was coming into contact with the endothelium of the muscular MA vessels, a solution of 10-5 M bradykinin was also infused into their lumen. Two fluorescence measurements were then taken 20 min apart. At the end of these experiments, 10-3 M phenylephrine was added to the bath to ensure a contraction of the vessel was seen and that the endothelium was intact and viable.
Statistical Analysis
Results are presented as a mean ± standard error. Data were analyzed by Student t-test and significance was considered at P < 0.05.
RESULTS
The MA and MV extraluminal fluorescence was directly proportional to the intraluminal pressure increase (Figure 2). For each vessel this increase was only significant at 300% of their respective physiological pressures (P<0.01). At all pressure ranges studied the MV had a significantly higher permeability when compared to the MA (Figure 2).
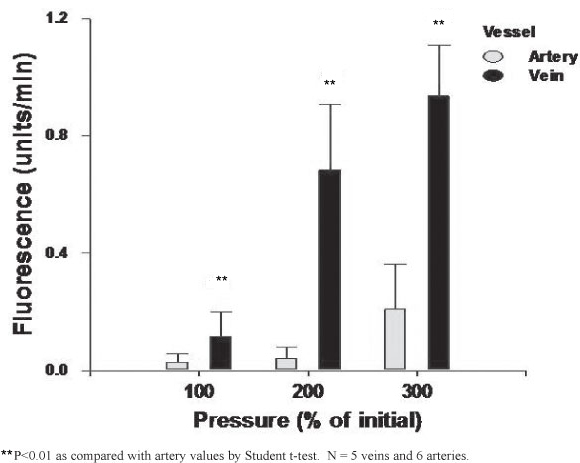
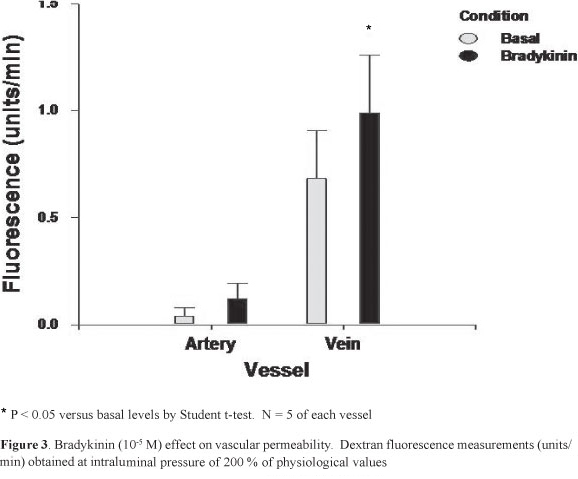
Figure 3 shows the MV and MA permeability to dextran following incubation with 105 M of bradykinin at 200% of physiological pressures. Whereas only a tendency for an increase was observed for the MA, the MV permeability was significantly greater following bradykinin exposure (P < 0.05). At basal and following bradykinin exposure the MV permeability was significantly higher, when compared with the MA values (P<0.01)
DISCUSSION
A significantly greater vascular permeability was documented in the rat mesenteric veins, as compared with the adjacent arteries. In vitro exposure to bradykinin, a compound believed to be involved in inflammation-related vascular leakage, only increased MV permeability. These results suggest that as compared with arteries, inflammation-induced changes in permeability are more likely to occur on the venous side of the mesenteric vasculature.
Vascular permeability occurs as a result of paracellular or transcellular transport across the vessel wall. The former occurs via endothelial wall gaps, whereas transcellular transport is characterized by molecule movement across the cells. In order to evaluate the pressure-dependent changes in vascular permeability we utilized dextran. This molecule, when present in the intraluminal space, does not cross an intact arterial vessel wall maintained at physiological pressure. Yet, this methodology cannot distinguish how the dextran was transported across the vessel.
Paracellular transport is likely to have played a significant role in the pressure-dependent increase in MA and MV permeability since increased pressures even with a fixed number of filtration slits open would lead to greater dextran extravasation. It has been suggested, however, that the size and number of endothelial gaps does not remain constant and may change under different conditions9.
To the best of our knowledge, limited data are available on the mesenteric vessel permeability induced by in vitro changes in intravascular pressure. Cipolla et al have shown that increasing the posterior cerebral arterial intravascular pressure in rats increases pinocytosis8. Thus a similar increase in transcellular transport might be operative in the MA and MV evaluated in the present study.
MV was significantly more permeable at 200% and 300% pp compared to MA at the same physiological pressure. This was possibly due to the MVs having thinner walls with less smooth muscle to impede permeability via paracellular transport compared to MA. However it is equally possible that since increased pressure increases transcellular transport, this increase might vary in different types of vessels and may also contribute to the change between MA and MV permeability at pressures above physiological. Even at physiological pressures, the MV showed a higher permeability when compared with the MA in the present study.
Bradykinin has been previously shown to only induce an increase in permeability in postcapillary venules7. The present data confirm these previous observations showing that bradykinin-induced increased permeability was only observed in the MV. Previous studies in postcapillary venules suggest that the changes in permeability induced by bradykinin occur through increased inter-endothelial gap formation6,10.
Previous studies addressing the bradykinin in vivo effect on vascular compartments indicated that this compound causes leukocyte tissue infiltration 11-13. Leukocytes have been shown to directly increase endothelial permeability14. In the current study, the MV bradykinin-induced permeability was studied in vitro and in the absence of leukocytes. Our results suggest that bradykinin appears to have a direct effect on the MV wall that is responsible for the increase in permeability. Such an effect is likely secondary to the bradykinin-induced nitric oxide release, since endothelial-derived nitric oxide release has been implicated in vascular permeability15.
Lastly we utilized the mesenteric resistance vessels to evaluate vascular permeability as it relates to bowel disease. These vessels, although participating in the regulation of blood flow to the bowel, are not the only ones likely involved in peritoneal fluid accumulation. Further studies attempting to address other vessels involved in this process are warranted. Similarly we utilized the adult rat to evaluate mesenteric vessel permeability. Although there is no evidence that the permeability of these vessels is age-dependent, there is a need to obtain similar measurements earlier in life utilizing larger animals where proper evaluation of the mesenteric vascular bed is feasible.
In conclusion, we have shown that when comparing rat mesenteric arteries, vascular permeability mostly happens at the venous side and is enhanced by bradykinin. These data highlight the importance of the venous circulation on edema peritoneal fluid formation. Further studies addressing the factors accounting for the increased vascular permeability of venous, as compared with arterial vessels, is warranted for the further understanding of abdominal fluid accumulation.
REFERENCES
1. Bill A, Linder J. Sympathetic control of cerebral blood flow in acute arterial hypertension. Acta Physiol Scand; 96:114-21;1976 [ Links ]
2. Medeiros R, Cabrini DA, Ferreira J, Fernandes ES, Mori MA, Pesquero J.B. et al. Bradykinin B1 receptor expression induced by tissue damage in the rat portal vein: a critical role for mitogen-activated protein kinase and nuclear factor-kappaB signaling pathways. Circ Res; 94:1375-82;2004 [ Links ]
3. Regoli D, Marceau F, Barabe J. De novo formation of vascular receptors for bradykinin. Can J Physiol Pharmacol; 56:674-7;1978 [ Links ]
4. Adamson RH, Ly JC, Fernandez-Miyakawa M, Ochi S, Sakurai J, Uzal F. et al. Clostridium perfringens epsilon-toxin increases permeability of single perfused microvessels of rat mesentery. Infect Immun; 73:4879-87;2005 [ Links ]
5. Baldwin AL, Thurston G, Al NH. Inhibition of nitric oxide synthesis increases venular permeability and alters endothelial actin cytoskeleton. Am J Physiol; 274:H1776-H1784;1998 [ Links ]
6. Feletou M, Bonnardel E. Canet E. Bradykinin and changes in microvascular permeability in the hamster cheek pouch: role of nitric oxide. Br J Pharmacol; 118:1371-6;1996 [ Links ]
7. Svensjo E, Arfors KE. Dimensions of postcapillary venules sensitive to bradykinin and histamine-induced leakage of macromolecules. Ups J Med Sci; 84:47-60;1979 [ Links ]
8. Cipolla MJ, Vitullo L, De Lance N, Hammer E. The cerebral endothelium during pregnancy: a potential role in the development of eclampsia. Endothelium; 12:5-9; 2005 [ Links ]
9. Baldwin A.L. Modified hemoglobins produce venular interendothelial gaps and albumin leakage in the rat mesentery. Am J Physiol; 277:H650-H659;1999 [ Links ]
10. Mayhan WG. Role of nitric oxide in modulating permeability of hamster cheek pouch in response to adenosine 5'-diphosphate and bradykinin. Inflammation; 16:295-305;1992 [ Links ]
11. Koyama S, Rennard SI, Robbins RA. Bradykinin stimulates bronchial epithelial cells to release neutrophil and monocyte chemotactic activity. Am J Physiol; 269:L38-L44;1995 [ Links ]
12. Satoh K, Yoshida H, Imaizumi TA, Koyama M, Takamatsu S. Production of platelet-activating factor by porcine brain microvascular endothelial cells in culture. Thromb Haemos; 74:1335-9;1995 [ Links ]
13. Shigematsu S, Ishida S, Gute DC, Korthuis RJ. Concentration-dependent effects of bradykinin on leukocyte recruitment and venular hemodynamics in rat mesentery. Am J Physiol; 277:H152-H160;1999 [ Links ]
14. Dull RO, Garcia JG. Leukocyte-induced microvascular permeability: how contractile tweaks lead to leaks. Circ Res; 90:1143-4;2002 [ Links ]
15. Bucci M, Roviezzo F, Posadas I, Yu J, Parente L, Sessa WC. et al. Endothelial nitric oxide synthase activation is critical for vascular leakage during acute inflammation in vivo. Proc Natl Acad Sci USA; 102: 904-8;2005 [ Links ]
 Address for Correspondence:
Address for Correspondence:
Dr. Jaques Belik
professor of pediatrics
University of Toronto
Division of Neonatology
Hospital for Sick Children
room 3886 555 University Ave Toronto
Ontario, M5G 1X8 - Canada
Phone (416) 813-2165 Fax (416) 813-5245
E-mail: Jaques.Belik@SickKids.caIvanecky
Recebido em 02/02/10
Modificado em 06/07/10
Aceito em 12/08/10