Serviços Personalizados
artigo
Indicadores
Compartilhar
Journal of Human Growth and Development
versão impressa ISSN 0104-1282
Rev. bras. crescimento desenvolv. hum. vol.23 no.3 São Paulo 2013
ORIGINAL RESEARCH
Preterm infant language development: a role for breast milk fatty acids
Tatiana Toro-RamosI; Maria Dalva Barbosa Baker MéioII; Denise Streit MorschII; Maria Elisabeth Lopes MoreiraII; Maria das Graças Tavares do CarmoIII; Rosely SichieriIV; Daniel J. HoffmanV
INew York Obesity Nutrition Research Center, St. Luke's-Roosevelt Hospital and Institute of Human Nutrition, Columbia University College of Physicians and Surgeons, New York
IIDepartment of Neonatology, Fernandes Figueira Institute/Fiocruz
IIIJosué de Castro Nutrition Institute, Federal University of Rio de Janeiro
IVInstitute of Social Medicine, State University of Rio de Janeiro
VDepartment of Nutritional Sciences, Rutgers, The State University of New Jersey
ABSTRACT
Premature infants have an increased risk of developmental disabilities during infancy and childhood. A crucial period of fetal polyunsaturated fatty acid accretion bypassed with prematurity.
OBJECTIVE: to study how the fatty acid composition of breast milk in breast-fed premature infants is associated with cognitive, language, and motor development.
METHODS: participants included twenty-five healthy preterms, born adequate for gestational age at the Fernandez Figueira Institute, Rio de Janeiro, Brazil. Fatty acid composition of breast milk samples from the first week postpartum was analyzed using gas-liquid chromatography. Bayley-III developmental scales were applied at 9 or 12 months corrected age.
RESULTS: regression analyses revealed that the ratio of linoleic acid to alpha-linolenic acid was positively associated with receptive language development (â = 1.49, p = 0.03). Women with preterm infants showed breast milk long chain polyunsaturated fatty acids concentrations consistent with worldwide levels and a high ratio of linoleic acid to alpha-linolenic acid that might be beneficial for language development in the premature infant.
CONCLUSION: a higher ratio of linoleic to alpha-linolenic acid in breast milk could exert beneficial effects for receptive language development in preterm infants fed breast milk. Larger adequately powered longitudinal studies are recommended to better understand the breast milk composition of this population and its association to developmental indices during infancy.
Key words: fatty acids, essential, cognition, language, breastfeeding, preterm infants.
INTRODUCTION
Approximately 11.1% of births worldwide, 9.2% of births in Brazil, and 12.0% of births in North America occur before the full gestational period1 (<37 weeks gestation). Premature infants, in particular boys, have an increased risk of disabilities, such as motor impairment, learning disabilities, and speech and language delay during infancy and childhood2 resulting from various nutrient deficiencies3. The third trimesters of gestation is a vulnerable period for nutritional insults, such as essential fatty acids (EFA) and long chain polyunsaturated fatty acids (LCPUFA), necessary for optimal development of the central nervous system (CNS) and organ membranes4. Maternal LCPUFA are delivered to the fetus during the third trimester of pregnancy and to newborns during breastfeeding when they are needed for development of the central nervous system, retinal cells, and organs4. Yet, what data exist on infant feeding following premature births and later cognition mainly come from studies focused on supplemented formulas and breastfeeding, and few examine the composition of the breast milk, but none have studied its relationship to cognitive, language, and motor development.
Optimal maternal EFA and LCPUFA status are essential prenatally and during breastfeeding for proper brain maturation, development, and visual acuity of premature infants4. However, the role of LCPUFA on cognition is unclear, as only a borderline positive association was found for preterm infants5. With premature delivery, the LCPUFA supply is interrupted, placing a newborn at risk for developmental impairments. However, whether premature infants benefit from breast milk is unclear. A review of randomized clinical trials (RCT) found that mental development for preterm infants fed LCPUFA-supplemented formula was significantly greater compared to control groups6. Moreover, there was no clinically significant effect of high-DHA supplemented formula on language, behavior, and temperament using the MacArthur Communicative Development Inventory7 or between maternal DHA supplementation and neurodevelopmental scores assessed by the Bayley-III8. Differences in the results may be related to the use of global, rather than specific areas of cognition tests or a gender effect in which only girls were found to have positive response to LCPUFA supplementation9. Nevertheless, various supplementation forms and the cognitive protocols tend weaken any broad conclusions that may be drawn from these studies.
Presently, the adequate intake (AI) of DHA for pregnant and lactating women is 200-300 mg/day DHA10, with an adequate ratio of LA to ALA of d" 4:1 for appropriate interconversion of ALA to EPA and DHA11. Women with premature infants have significantly greater concentrations of breast milk LCPUFA during the first week postpartum compared to women with full-term births12. While studies that compare developmental outcomes between formula-fed and breastfed infants are limited, one study found that breastfeeding might be advantageous over formula for preterm infants13. We hypothesize that breast milk LCPUFA reflective of high maternal intake benefit preterm infant developmental indices score. Therefore, the aim of the study was to evaluate the association between breast milk EFA and LCPUFA and cognitive, language, and motor functions according to Bayley-III scores among preterm newborns from Brazil at one year of age.
METHODS
Study design and subjects
The sample of this preliminary cohort study consisted of 25 healthy preterm infants born adequate for gestational age at the Instituto Fernandes Figueira (IFF) between March 2005 and November 2007. This study followed the ethical guidelines established by the Declaration of Helsinki. The Institutional Review Board/ Research Ethics Committee of the IFF (CAAE 0050.0.008.000-04) approved the study and written informed consent was obtained from the children's parents or guardians.
Mother's milk sample collection
Breast milk was manually expressed after breastfeeding in the morning between the 2nd and 7th day postpartum. A minimum of 1 ml of breast milk was collected from each subject in Eppendorf tubes. All samples were stored in a freezer at 4°C for no more than 5 days before being transported on ice to the laboratory where they were stored at - 70°C until analysis.
Fatty acid analysis
Breast milk samples were analyzed for total lipid fatty acid using gas-liquid chromatography (GLC) as described previously14. Lepage and Roy's15 transesterification method was used to prepare the fatty acid methyl esters (FAME). FAME in a PerkinElmer chromatography autosystem with a hydrogen flame ionization detector and a capillary column (60m x 0.30mm i.d.) packed with 10% SP 2330 (Supelco Inc, Bellefonte, PA, USA) as a stationary. Injection and detection temperatures were both set at 220°C and oven temperature was programmed to vary 5°C/min from 40 to 225 °C. Esters were identified by comparing retention times with known standards (Sigma Aldrich, St Louis, MO, USA) and quantification was done by calculating peak areas with an integrator. Results were expressed as relative area percentage of total FAME.
Bayley Scales of Infant and Toddler Development, Third Edition (Bayley-III)
Trained psychologists applied the Bayley-III at 9 or 12 months corrected for gestational age in a separate room with the infant and the parent or guardian. After explaining the process of the test, each parent was interviewed regarding the daily care routine and home environment. Raw scores were based on the corrected age of the infant16. In comparison to previous Bayley test editions, the Bayley-III allows separating global language scores into receptive and expressive language and motor scores into fine and gross motor. The Social-Emotional and Adaptive Behavior Scales were not used, as these were developed in American populations and cultural and social differences would have led to invalid results.
Data analysis
DHA and LA/ALA values were skewed and were log transformed, thus data are shown on an exponentiated transformed scale. The Bayley-III scores at 12 mo of age were used except for those infants for whom Bayley-III scores were available from 9 mo of age only (n=8). No significant differences were found between scores at 9 or 12 months.
Multiple linear regression analyses evaluated the association between fatty acid exposures and developmental indices outcomes, adjusting for sex, gestational age, breastfeeding duration and age at testing (9 or 12 months corrected age). Standardized beta coefficients were calculated as the difference between each observed value and the respective mean divided by the standard deviation for the dependent and each independent variable.
The initial multiple regression analysis model was performed with each FA alone and subsequently adding the confounding variables, sex, gestational age, breastfeeding duration, and age at testing. Other potential confounders, such as maternal ethnicity were not included in the regression model due to the small sample size. A sensitivity analysis was conducted to evaluate whether inclusion or exclusion of outliers [values that fell under the 5th (n=1) or above the 95th percentiles (n=2)] changed the primary results. Based on previous research17 evaluating similar parameters (Cognitive and Language scores of the Bayley III exam), differences of 1-2 units with a standard deviation of 0.4 to 0.6 was used to determine a sample size of 18 with 90% power to detect differences or associations with Type I error of 0.05. As we were evaluating the association between exposure and outcomes as a group, predicting for sample loss, we estimated that a sample size of 25 would be sufficient to detect a correlation coefficient of 0.4 with a statistical significance set at p = 0.05. All statistical analyses were completed using SAS version 8.0 (SAS Institute, Cary, NC, USA) and statistical significance was determined at p = 0.05.
RESULTS
A total of 25 mothers with premature infants born < 33 weeks gestational age participated in the study at 9 or 12 months postpartum and provided a breast milk sample during the first week postpartum (Figure 1). Cohort characteristics are presented in Table 1. Two infants were exclusively breastfed during follow-up. The average length of exclusive breastfeeding was 4 months of age and 6 mothers reported breastfeeding for more than 6 months. Others received both breast milk and formula. Duration of breastfeeding (4.1 ± 4.6 months for boys, 3.7 ± 4.3 months for girls) and gestational age (29.6 ± 2.8 weeks for boys, 29.6 ± 3.0 weeks for girls) did not vary by sex. Seventy-six percent of the infants received respiratory support at the NICU. There were also no significant differences between the Bayley scores of boys and girls (Table 2).

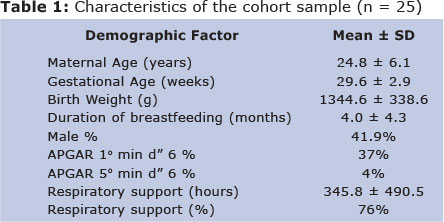
Mean content of LA, ALA, AA, and DHA as percentage of total fatty acids in the sample from the first week postpartum, and the ratio of LA to ALA (23 to 1) are summarized in Table 3. The proportion of LA was the highest of all PUFA in the breast milk samples. Multiple linear regression analyses revealed that the ratio of LA to ALA was positively associated with receptive language development (â= 1.49, p = 0.03) after adjusting for sex, gestational age, length of breastfeeding, and duration of follow-up (Table 4). We found that for every one unit change in LA/ALA, there is a 1.49 unit increase in the standardized value for Receptive Language assessment.

COMMENT
The role of breast milk LCPUFA on sensitive areas of development for preterm infants is unclear. We examined the relationship between infant breastfeeding following premature birth and later cognition, language, and motor development. We found that breast milk from the first week postpartum contained an elevated ratio of LA to ALA that was positively associated with measures of receptive language. Our study found similar percentages of LCPUFA in the milk from mothers of preterm infants to a previous study conducted in Brazil18 and higher values compared to other studies in different countries19. Specifically, the values of AA in the present study (0.4%) were slightly above the worldwide mean value of 0.32% (SD 0.22)20. Although no other significant associations were observed, possibly due to limitations of the sample size, findings suggest the importance of educating post-partum women on the developmental benefits of preterm infant breastfeeding and possible tailored fortification of breast milk according to individual nutritional needs21.
We found that the ratio of LA to ALA was a positive predictor of receptive language. A high dietary ratio of n-6 to n-3 fatty acids is associated with a higher risk for chronic disease later in life22, and excessive LA might have a negative impact on neurodevelopment23. The ideal dietary ratio of LA to ALA for achieving 20% higher plasma and erythrocyte levels of eicosapentaenoic acid (EPA), precursor of DHA is 4:122. In our study, the breast milk ratio of LA to ALA was 23 to 1, which might explain the higher AA levels. This is in agreement with observations of variable ratios of LA to ALA in colostrum from mothers of preterm and full-term infants18. Excess LA downregulates ALA metabolism into its LCPUFA because it competes with ALA for desaturation24. Therefore, maternal diet of preformed LCPUFA may be a better source for the infant, and when the diet lacks LCPUFA, an adequate LA to ALA ratio is critical to sustain an efficient conversion rate into EPA and DHA for neural tissue and retinal accretion, and adequate levels of AA for growth25. Nevertheless, based on our results, the possibility that a high LA/ALA ratio might benefit premature infant language development should be further examined.
Language is a particular area of development that may be delayed in preterm infants26, 27. The factors that affect language development are not completely understood. Breast milk was not associated with expressive language (Bayley-II), but maternal education was associated, and no differences were found between formula- and breast-fed term infants on tests of receptive vocabulary (Peabody Picture Vocabulary Test-Revised) and expressive vocabulary (mean length of utterance)28. Moreover, no evidence of improved language development was observed between infants fed 1% DHA versus 0.3% DHA breast milk using the MacArthur Communicative Development Inventory (MCDI)7. Confounding factors such as socioeconomic status and maternal education need to be taken into consideration. In particular, prolonged respiratory support in the neonatal intensive care unit (NICU) is known to affect oral growth and negatively impact infant speech development29. In this study, time of respiratory support did not influence the results. No significant associations between breast milk fatty acids and cognitive and motor indices of development were observed in our study. Possible differences between studies may be due to a small sample size, different levels of supplementation and LCPUFA in breast milk, and to commonly unconsidered factors, such as maternal education and the length of intubation in the NICU of preterm infants.
Gender differences have been previously observed in studies of preterm infants randomized to receive DHA supplemented formula versus non-supplemented formula)9, increasing general mental developmental index (MDI) scores (Bayley-II), for girls only30. Although gender effects of LCPUFA formula supplementation have been shown9, we were not able to see gender differences in adjusted analyses due to our small sample size.
Our findings of a high n-6/n-3 ratio from our study are in agreement with a previous review of studies in the Brazilian population on the increased consumption of LA and the decreasing consumption of DHA31. Our study possibly did not capture this due to a small sample size, thus, future studies should include larger sample sizes of women with varied fatty acid intakes to allow for more generalizable results. Some limitations in our study include lack of dietary information and a limited sample size. Null results as well as significant ones thus merit equal consideration. Larger longitudinal studies that control for additional confounders, such as ethnicity, socioeconomic status, maternal education, and the maternal diet during pregnancy will lead to a clearer picture of the breast milk composition of this population of women and its association to developmental indices during infancy. Clinical and public health efforts should give careful attention to overall nutritional needs and LCPUFA in breast milk to avoid deficiencies and to individualized supplementation of breast milk considering infant gender to optimize premature infant development.
In conclusion, a high linoleic acid to alpha-linolenic acid ratio in breast milk seems to be beneficial for receptive language development in breastfed premature infants. A larger sample is necessary to detect possible differences in indices of development between preterm males and females and to further evaluate the associations in the present study.
AKNOWLEDGEMENTS
We thank all participating infants and their mothers for their collaboration. Felipe Domingues assisted with the gas-liquid chromatography analyses.
Conflict of Interest Disclosures: Authors report no declarations of interest.
REFERENCES
1. Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012, 379 (9832): 2162-72. [ Links ]
2. Bhutta AT, Cleves MA, Casey PH, Cradock MM, Anand KJ. Cognitive and behavioral outcomes of school-aged children who were born preterm: a meta-analysis. JAMA. 2002,288(6):728-37. [ Links ]
3. Shah MD, Shah SR. Nutrient deficiencies in the premature infant. Pediatr Clin North Am. 2009, 56(5): 1069-83. [ Links ]
4. Al MD, van Houwelingen AC, Kester AD, Hasaart TH, de Jong AE, Hornstra G. Maternal essential fatty acid patterns during normal pregnancy and their relationship to the neonatal essential fatty acid status. Br J Nutr. 1995,74(1):55-68. [ Links ]
5. Makrides M, Collins CT, Gibson RA. Impact of fatty acid status on growth and neurobehavioural development in humans. Matern Child Nutr. 2011, 7 (Suppl 2): 80-8. [ Links ]
6. Smithers LG, Gibson RA, McPhee A, Makrides M. Effect of long-chain polyunsaturated fatty acid supplementation of preterm infants on disease risk and neurodevelopment: a systematic review of randomized controlled trials. Am J Clin Nutr. 2008,87(4):912-20. [ Links ]
7. Smithers LG, Collins CT, Simmonds LA, Gibson RA, McPhee A, Makrides M. Feeding preterm infants milk with a higher dose of docosahexaenoic acid than that used in current practice does not influence language or behavior in early childhood: a follow-up study of a randomized controlled trial. Am J Clin Nutr. 2010,91(3):628-34. [ Links ]
8. Makrides M, Gibson RA, McPhee AJ, Yelland L, Quinlivan J, Ryan P, et al. Effect of DHA supplementation during pregnancy on maternal depression and neurodevelopment of young children: a randomized controlled trial. JAMA. 2010,304(15):1675-83. [ Links ]
9. Isaacs EB, Ross S, Kennedy K, Weaver LT, Lucas A, Fewtrell MS. 10-year cognition in preterms after random assignment to fatty acid supplementation in infancy. Pediatrics. 2011,128(4):e890-8. [ Links ]
10. Makrides M. Is there a dietary requirement for DHA in pregnancy? Prostaglandins Leukot Essent Fatty Acids. 2009,81(2-3):171-4. [ Links ]
11. Makrides M, Gibson RA. Long-chain polyunsaturated fatty acid requirements during pregnancy and lactation. Am J Clin Nutr. 2000,71(1 Suppl):307S-11S. [ Links ]
12. Kovács A, Funke S, Marosvölgyi T, Burus I, Decsi T. Fatty acids in early human milk after preterm and full-term delivery. J Pediatr Gastroenterol Nutr. 2005,41(4):454-9. [ Links ]
13. Maayan-Metzger A, Avivi S, Schushan-Eisen I, Kuint J. Human milk versus formula feeding among preterm infants: short-term outcomes. Am J Perinatol. 2012,29(2):121-6. [ Links ]
14. Azara CR, Maia IC, Rangel CN, Silva-Neto MA, Serpa RF, De Jesus EF, et al. Ethanol intake during lactation alters milk nutrient composition and growth and mineral status of rat pups. Biol Res. 2008,41(3):317-30. [ Links ]
15. Lepage G, Roy CC. Direct transesterification of all classes of lipids in a one-step reaction. J Lipid Res. 1986,27(1):114-20. [ Links ]
16. Bayley N. Bayley Scales of Infant and Toddler Development, Third Edition (Bayley-III). San Antonio, TX: Harcourt Assessment, 2006. [ Links ]
17. Lowe JR, Duncan AF, Bann CM, Fuller J, Hintz SR, Das A, et al. Early working memory as a racially and ethnically neutral measure of outcome in extremely preterm children at 18-22months. Early Hum Dev. 2013. [ Links ]
18. Tinoco SM, Sichieri R, Setta CL, Moura AS, do Carmo MG. Trans fatty acids from milk of Brazilian mothers of premature infants. J Paediatr Child Health. 2008,44(1-2):50-6. [ Links ]
19. Kuipers RS, Fokkema MR, Smit EN, van der Meulen J, Boersma ER, Muskiet FA. High contents of both docosahexaenoic and arachidonic acids in milk of women consuming fish from lake Kitangiri (Tanzania): targets for infant formulae close to our ancient diet? Prostaglandins Leukot Essent Fatty Acids. 2005,72(4):279-88. [ Links ]
20. Brenna JT, Varamini B, Jensen RG, Diersen-Schade DA, Boettcher JA, Arterburn LM. Docosahexaenoic and arachidonic acid concentrations in human breast milk worldwide. Am J Clin Nutr. 2007,85(6):1457-64. [ Links ]
21. Di Natale C, Coclite E, Di Ventura L, Di Fabio S. Fortification of maternal milk for preterm infants. J Matern Fetal Neonatal Med. 2011,24 Suppl 1:41-3. [ Links ]
22. Simopoulos AP. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Biol Med (Maywood). 2008,233(6):674-88. [ Links ]
23. Novak EM, Dyer RA, Innis SM. High dietary omega-6 fatty acids contribute to reduced docosahexaenoic acid in the developing brain and inhibit secondary neurite growth. Brain Res. 2008,1237:136-45. [ Links ]
24. Gibson RA, Muhlhausler B, Makrides M. Conversion of linoleic acid and alpha-linolenic acid to long-chain polyunsaturated fatty acids (LCPUFAs), with a focus on pregnancy, lactation and the first 2 years of life. Matern Child Nutr. 2011,7 (Suppl 2):17-26. [ Links ]
25. Innis SM. Omega-3 Fatty acids and neural development to 2 years of age: do we know enough for dietary recommendations? J Pediatr Gastroenterol Nutr. 2009,48 (Suppl 1):S16-24. [ Links ]
26. Bosch L. Precursors to language in preterm infants: speech perception abilities in the first year of life. Prog Brain Res. 2011,189:239-57. [ Links ]
27. Barre N, Morgan A, Doyle LW, Anderson PJ. Language abilities in children who were very preterm and/or very low birth weight: a meta-analysis. J Pediatr. 2011,158(5):766-74 e1. [ Links ]
28. Auestad N, Scott DT, Janowsky JS, Jacobsen C, Carroll RE, Montalto MB, et al. Visual, cognitive, and language assessments at 39 months: a follow-up study of children fed formulas containing long-chain polyunsaturated fatty acids to 1 year of age. Pediatrics. 2003,112(3 Pt 1):e177-83. [ Links ]
29. Capilouto GJ, Desai N, Winner R, Caldwell R, Bada H. Orotracheal intubation in the NICU and expressive language outcomes at 24-30 months. J Med Speech Lang Pathol. 2008,16(3):157-73. [ Links ]
30. Makrides M, Gibson RA, McPhee AJ, Collins CT, Davis PG, Doyle LW, et al. Neurodevelopmental outcomes of preterm infants fed high-dose docosahexaenoic acid: a randomized controlled trial. JAMA. 2009,301(2):175-82. [ Links ]
31. Torres AG, Trugo NM. Evidence of inadequate docosahexaenoic acid status in Brazilian pregnant and lactating women. Rev Saude Publica. 2009,43(2):359-68. [ Links ]
 Corresponding author:
Corresponding author:
ttororamos@chpnet.org
Manuscript submitted Sept 28 2013.
Accepted for publication Oct 15 2013.













