Services on Demand
article
Indicators
Share
Journal of Human Growth and Development
Print version ISSN 0104-1282
Rev. bras. crescimento desenvolv. hum. vol.24 no.2 São Paulo 2014
ORIGINAL RESEARCH
Association among sexual maturation, overweight and central adiposity in children and adolescents at two schools in São Paulo
Jéssica Rodrigues de OliveiraI; Maria Fernanda Petroli FrutuosoII; Ana Maria Dianezi GambardellaIII
IDoutoranda, Programa de Nutrição em Saúde Pública, Faculdade de Saúde Pública, Universidade de São Paulo, Av. Dr Arnaldo, 715 - 01246-904, São Paulo, Brasil
IIDepartamento de Ciências da Saúde, Universidade Federal de São Paulo, Av. Ana Costa, 95 - 11060-001, Santos, Brasil
IIIDepartamento de Nutrição, Faculdade de Saúde Pública, Universidade de São Paulo, São Paulo, Brasil, Av. Dr Arnaldo, 715 - 01246-904, São Paulo, Brasil
ABSTRACT
The objective of this study was to assess the association among sexual maturation (SM), overweight and central adiposity in children and adolescents. A total of 617 children and adolescents age 8 to 18 years old participated in the longitudinal study. Three samples were collected including data on weight, height and waist circumference. Overweight was classi-fied based on critical values for body mass index (BMI) adopted for Brazilian children and teenagers. SM stage (SMS) was done by self-assessment in three children and adolescent samples. Participants were distributed in quartiles based on SMS and sex. Accelerated-maturing subjects were compared to others. Data was analyzed by linear regression and logistic regression. We found a negative association between BMI z-score and onset of SM in boys, whereas for girls relatively-accelerated SM was positively associated to overweight and values of BMI z-score. Relatively-accelerated maturing girls showed more central adi-posity. The research concluded that accelerated SM was associated to overweight and high-er increase in BMI for both sexes, highlighting the importance of identifying SMS in nutri-tional assessment for children and teenagers.
Key words: adolescents, precocious puberty, sexual development, body mass index, overweight, central adiposity.
INTRODUCTION
Currently Brazil has a high number of obese individuals in almost every age range, with the highest concentration among those with lower incomes. Projections done in the beginning of the last decade by the World Health Organization (WHO) identified obesity as a major issue for public health and, since then, it has gradually captured the attention of specialists worried by its growing incidence¹.
The secular trend of an increase in obesity rates has been observed in Europe (England, Finland, Germany, Netherlands, Sweden and others) and the Central Pacific area (Australia and Samoa). In Africa and Asia, obesity has had lower rates and has become more common within urban populations¹.
Several studies around the world concerning obesity address adolescents because of biopsychosocial changes occurring at this life stage, as well as the proximity to biological maturation, which allows for opportunities to prevent health problems in adult age.
In 2002, the Pan American Health Organization (PAHO) showed that one quar-ter of children and adolescents in Latin American countries such as Chile, Peru and Mexico were overweight2. More recent data noted that overweight has doubled in North-American adolescents in the last two decades3,4. In Brazil, overweight has tripled in the last 30 years among adolescents age 10 to 19 years old5-7.
Body mass index (BMI) is largely used in nutritional assessment of individuals and populations because of low cost and convenience when compared to other methods. At the present time, different criteria for nutritional classification of adolescents are based on BMI values according to age and sex, and not with SMS, although it better reflecte the stage of development within this population. Furthermore, changes occurring during puber-ty cause anthropometric trans-formations and changes in adolescents' body composition and have a high impact on nutritional stage8.
Pubertal development follows physiological chronology of events owing to modifications on some hormone secretion patterns. The activation of the hypothalamo-hypophyseal-gonadal axis starts, under stimulus of gonadotropin, the secretion of sexual steroids (predominantly testosterone in boys and estradiol in girls), which are in charge of morphological changes in the puberal period. Production of such hormones results in the appearance of secondary sex characteristics defining puberty onset9. The effect of changes during the beginning of puberty also causes a contrast in emotional development according to sex10.
In the 1970s, Frish and Revelle11 used population studies on the age at me-narche to suggest that weighing about 48 kilograms is needed for normal development and the first menstrual cycle (critical weight hypothesis). Since then, research studies all over the world have been trying to associate SM process with anthropometric variations and nutritional status, but outcomes remain controversial.
Early puberty (EP) has been defined as the onset of secondary sex characteristics before 8 years old in girls and 9 years old in boys12,13. Adair and Gordon-Larsen14, based on a sample study of 13 - to 19 - year - old girls from different ethnic groups in National the Longitudinal Study of Adolescent Health, observed that overweight was significantly higher among EP adolescents. EP doubled the risk of developing overweight among these teenagers.
Nevertheless, a limited number of studies have been conducted with boys to verify the relationship between EP and obesity. This fact may be attributed basically to difficulty in measuring SM in boys within epidemiological studies. Age at menarche is the most common SM indicator in population studies because of convenience and low cost. On the other hand, younger girls and boys belonging to all age ranges cannot be included in the studies. According to some authors, this method still leads to estimation mistakes as the informed age at menarche may be wrong, especially when it happened a long time before the question is asked15,16.
Identifying and comprehending the influence of sexual maturation in developing obesity is essential for formulating prevention policies and treatment in this age range, besides assisting with nutritional status assessment of children and adolescents. Thus, our goal is to verify the influence of relatively accelerated-SM on overweight and central adi-posity in children and adolescents.
METHODS
A longitudinal study was conducted with 8 to 18-year-old students from both sexes. They participated in three data collections during one year, with a six-month break between them. Collections were done in the following order: first one, August/September 2001; second one, March/April 2002; and the third one, September/October 2002. Individ-uals were selected from one public school and one private school in the city of São Paulo with the aim of recruiting participants from different socioeconomic populations.
Sample size was calculated by considering a level of significance of 95% and 80% sample power (1-2). Based on previous studies, a 25% prevalence of individuals with EP was estimated, with an OR value of 2 relating to output (obesity). Thus, the sample size to fit the estimated criteria was 605 individuals. An extra 10% was added to compensate for possible sample losses.
A single researcher completed the data collection utilizing interviews and anthropometric measurement at the schools. Data was registered in previously tested forms. Educational level was measured in completed years of study using surveys sent to the par-ents together with Consent Forms.
Age was calculated in months, considering the difference between born age and the interview date. Body weight measurement was done with a platform-style electronic scale with a capacity of 150 kg and precision of 100 g (Tanita TFB-521®). Height was measured by a stadiometer (Seca®) attached to the wall with a scale in millimetres (mm). Waist perimeter was measured at the end of expiration, with non-elastic webbing and scale in millimetres (mm), from the middle point between the iliac crest and the external part of the rib. Two height measurements and three waist perimeter measurements were completed. We considered the median of obtained values, as proposed by Lohman et al.17 Calculating BMI required dividing weight by the square of the height of each subject.
Overweight and obesity research was verified based on BMI critical values for Brazilian children and adolescents. Proposed by Conde and Monteiro18, this criterion is based on curves that take into account classical indicators for adult population and affect lower ages, built from original data dating from 1989 and belonging to the National Research on Health and Nutrition (Pesquisa Nacional de Saúde e Nutrição - PNSN).
Sexual maturation status was checked by self-assessment. Subjects were given images of five stages characterizing sexual development in children and adolescents ac-cording to Tanner stages19. Pubic hair stages (P1, P2, P3, P4, P5) were evaluated in both sexes, according to characteristics, amount and distribution. We considered genitalia stage (G1, G2, G3, G4, G5) for boys and breasts stage (M1, M2, M3, M4, M5) for girls, evaluated according to size, charac-teristics and shape. State 1 corresponds to growth and pre-pubertal development, whereas stages 2 to 4 correspond to progression from puberty to complete maturation (stage 5).
Subjects were distributed in age quartiles (months) adjusted to each Tanner stage and separated by sex. Thus relatively-accelerated maturation corresponded to those within the first age quartile for each SM stage during the three samples. Later the other quartiles were grouped and compared to the first quartile during the analysis.
Similar procedures were done for pubic hair and genitalia/breasts, with confor-mity between both criteria on classification of EP. Conformity and kappa value were 79.3% and 0.46 for boys and 86.1% and 0.64 for girls, respectively. Thus male genitals and female breasts were chosen for classifying the development stage in each sex, as they proved to be more reliable on detection of the hypothalamo-hypophyseal-gonadal axis, recommended by the WHO Expert Committee as international indicators for SM20.
Statistical analysis
Prevalence of overweight and obesity was evaluated according to sex and SM stage (relatively accelerated or not). A t-test was applied in order to verify differences in anthropometric measurements among mature subjects or non-early.
BMI z-score values were calculated for comparisons according to sex, age range and SMS. LMS parameters were applied. Such parameters were obtained from the construction of a BMI reference curve for the Brazilian population proposed by Conde and Monteiro (2006). By this method, data are concentrated in three smooth curves (L, M and S), specific for age. Curves M and S correspond to the mean and to BMI variation coeffi-cients in each layer. L parameter is the coefficient (Box-Cox) used for obtaining normal distribution of BMI values in each layer22. Thus, z-score values were calculated for BMI from the equation:
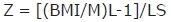
Logistic regression analysis was conducted in order to verify the relation be-tween relatively-accelerated maturation and overweight/obesity and later, using multiple linear regression, the association between relatively-accelerated SM, IMC and waist peri-meter. All analyses were stratified by sex and adjusted by age, height and mother's educa-tion. Collinearity was tested by examining the variance inflation factor (VIF). No variable showed perfect collinearity (VIF<10). In general, correlationship between variables was lower than 0.15. Statistical calculation was done with Stata 10.123 software and at a signi-ficance level of 0.05.
The present study is in accordance with Resolution #196, from 1996 October 10th of the Federal Council of Health in Brazil, which establishes rules for research studies involving human beings and has been approved by the Research Ethics Committee of the University of São Paulo's Faculty of Public Health.
RESULTS
Initially, data belonging to 650 children and adolescents were collected. From the third sample, 617 children and adolescents were followed: 40.7% (n = 251) boys and 59.3% (n=366) girls. Higher losses were observed in the male group-most of them due to stu-dents who dropped out of school.
The characteristics of the studied population are presented in Table 1. Boys showed higher weight gain and height in the observed period, with important statistical differences between sexes. Analyzing the students' nutritional stage in the beginning of the study, a major prevalence of overweight in girls was observed (27.4% vs. 23.8% among boys). In comparison, boys had a higher prevalence of obesity (8.5% vs. 7.3% among girls). A higher rate of overweight and obesity prevalence between collections was observed in boys.
Compared to those late maturing, relatively-accelerated maturing boys were significantly shorter (149.3 cm vs. 157.9 cm) and less heavy (46.8 kg vs. 51.8 kg) at the beginning of the study. As observed among boys, girls showing accelerated-maturity were significantly shorter (152.2 cm vs. 154.8 cm), but did not have significant differences in weight (48.1 kg vs. 49.5 kg). EM subjects from both sexes had higher weight and height gain during the period. Lower BMI values were also observed in EM boys. There were no statistical differences between sexes for waist perimeter values (Table 2).
Figure 1 shows BMI z-score means ccording to sexual development stage and sex. A decrease in z-score mean values among puberal and post-puberal boys is observed as age increases. Boys who started SM in earlier ages had higher BMI z-score values (2.67 DP).
On the other hand, boys who had not begun the process of SM showed mean values similar to BMI z-scores independently from age (0.93 DP in ages ranging from 8 to 10 years old; 0.81 DP in 10 to 12 years old; and 0.84 DP in 12 to 14 years old). In girls, this trend is not so evident, as puberal and post-puberal girls showed higher z-score values in younger ages (1.37 DP) and the lowest values are observed among girls ages 10 to 12 years old (0.94 DP). Prepuberal girls, as the others, had the lowest z-score values in ages ranging from 10 to 12 years old (0.05 DP).
Logistic regression analysis showed that relatively-accelerated SM is associated with an increase in overweight and obesity risk in both sexes. When adjusted by age, SM lost its significance as a risk factor for developing overweight in boys (Table 3).
Table 4 presents the multiple linear regression results. Adolescents with relatively-accelerated SM showed on average a higher increase in BMI z-score and in waist perimeter during the period of study with statistical significance only in girls.
DISCUSSION
Currently, BMI has been greatly applied for identification of adiposity in ado-lescents for its convenience, low cost and good correlation with body fat and being recom-mended by the WHO1. However, there is no consensus on the most proper BMI cut-off points for classifying nutritional status within this age range24. Thus, this study chose to use the national reference as it is represented by curves drawn from data originally belonging to research of national significance.
Another important point is the use of the LMS method for modelling curves, improving their randomness and is internationally better accepted, in addition to consider-ing the cut-off points applied to classifying overweight and obesity in adult age (BMI = 25 and 30 kg/m2, respectively) and affecting earlier ages25.
In the case of Brazilian children and adolescents, using centiles 85 and 95, instead of those based on adult BMI output, would involve obtaining higher values for the prevalence of obesity in girls, as well as higher prevalence of malnutrition, overweight and obesity in boys26.
Classifying SM raises some aspects for discussion. The first remark is on the self-assessment of the SM stage. The simplicity of the method, as well as obstacles for an objective analysis by the examiner led to a high number of research aimed at correlating self-assessment with the examination conducted by trained professionals. However, self-assessment does not show consensus in the literature in relation to the reliability of deter-mining SMS, as the method may lead to classification mistakes, intentionally or not. Na-tional and international studies note kappa values (concordance) between self-assessment and objective examination of 0.34 to 0.81 (48% to 86%) for girls and 0.29 to 0.88 (51% to 76%) for boys27-32.
Thus, in order to improve self-assessment accuracy, children and adolescents were distributed in small groups of the same sex and informed about adolescence, body development and SM. They also heard explanations on self-assessment and form filling, one-by-one. Information on the characteristics defining each SM stage was offered in order to improve self-assessment skills.
A second remark is on the classification of early SM. The classic cut-off points for puberty evaluation was based on the normal distribution of the age of SM onset and a confidence interval of 95% in epidemiologic studies done with British children 40 years ago12,13. However, incidence of early puberty differs a lot between both sexes. Many girls, but a small number of boys, showed secondary sexual characteristics development before 8 and 9 years old, respectively. Such difference may be attributed to several reasons as those inherent to sex and subjects' physiology, the trend in a decrease in SM onset, more evident in females, as is shown by a decrease in the menarcheal age in industrialized countries and those under development33.
Some authors and international institutions have proposed a review of these age parameters and the use of 25 percent in SM starting age distribution for the classification of EP33-35.
The lack of information referring to the onset of the SM process in all subjects blocked the use of existing cut-off points. In this sense, the present study, based on longitu-dinal design, chose to classify subjects with "relatively-accelerated maturation", done by age quartiles. As it is not a representative sample of the population, the status quo method could not be applied.
The inter and intra-individual differences relating to SM timing may also lead to mistakes in classifying early maturation, as a EP individual may show compensatory progression, thus not being classified as early in more advanced stages. Equally, non-early maturation subjects may show slower maturation progress and be classified as early in the next stage. Thus, for each collection, the division in age quartiles for each maturation stage according to sex was a possible method to be used when relatively-accelerated maturity was associated with subjects in the first age quartile for actual SMS in the three collections. The others were classified without relatively-accelerated maturity.
In this study higher BMI z-score values were observed in puberal or post-puberal youngsters unrelated to age and sex. This could be explained by the high increase in weight and height occurring during the process of SM. The onset of maturity in earlier ages was associated with higher BMI z-score values, mostly in boys.
For girls this study's findings reinforce conclusions reached by several national and international research studies: EP girls had higher weight and height values, as well as higher risk of overweight/obesity. Wang15 analyzing data from a representative sample of North-American children and adolescents ages 8 to 14 years old that participated in the Third National Health and Nutrition Examination Survey (NHANES III), conducted be-tween 1988 and 1994, observed that early SM is positively associated to the risk of over-weight (OR = 1.59 [1.05 - 2.42]) and obesity (OR=1.96 [1.11 - 3.47]) in girls.
Ribeiro et al.15, when studying Portuguese adolescents between 10 and 15 years old, verified that PP girls had a double chance of being overweight. Another study with African-American girls found a 3.6 higher risk of developing overweight among PP girls36.
When studying a sample of 13- to 19-year-old girls from different ethnicities, Adair and Gordon-Larsen14 observed that early SM doubled the risk of developing over-weight (BMI > percentile 85) in adolescents. Prevalence of overweight was significantly higher among adolescents showing early SM, regardless of ethnicity, with a higher occur-rence among black girls.
In contrast with the large number of studies noting the relationship between ob-esity and early maturation in girls, studies on boys are rare and differ in results. As with girls, PP Portuguese boys had double the chance of being overweight when compared to others16. On the other hand, Wang15 came to the opposite conclusion: EM was a protecting factor for overweight (OR = 0.65 [0.44 - 0.98]) and obesity (OR = 0.40 [0.20 - 0.82]) in boys.
Wang14 also verified that EP North-American girls had an average increase of 3.48 cm in height, 5.87 kg in weight and 1.50 kg/m2 in BMI. Similar values were found in this study, with an increase of 2.04 cm in height, 4.64 kg in weight and 1.44 kg/m2 in BMI. However, for boys opposite values were found compared to US samples (2.55 cm vs. - 2.47 cm in height; 0.45 kg vs. - 2.51 kg in weight; 0.61 kg/m2 vs. 0.98 kg/m2 in BMI). Although the influence of early SM in obesity goes in the same direction in both sexes, the influence of the anthropometric measurement increase differs for each sex.
Bratberg et al.37, in a longitudinal study conducted with 1,605 Norwegian ado-lescents, showed that the combination of central adiposity with EP raised the risk of over-weight in girls at the end of adolescence. Among boys no association was found.
The present study observed that boys and girls with relatively-accelerated ma-turity showed average higher values for waist perimeter (3.10 cm and 1.73 cm, respectively) at the end of the study, but with statistical significance only in females (p = 0.004). The increase in central adiposity in accelerated-maturing children and adolescents seems to be associated to other health risks. Recent studies show the association between EP and a rise in blood pressure38, hyperinsulinemia and resistance to insulin39. Nevertheless, these studies did not assess waist perimeter in children and adolescents to verify a possible association.
The onset and development of the whole SM process is influenced by genetic factors, mostly leading to individual variation of the pubertal phenomenon and by socio-environmental factors that should be positive for the maximum expression of adolescents' genetic potential. However, it is still unknown how such factors interact among themselves and influence the process of SM, and how the latter affects development of obesity. Thus, further research and longitudinal studies are necessary to follow subjects from childhood to adult age for a better comprehension of such relationships.
There is a positive association between relatively-accelerated SM in adolescents, more evident in girls. Although this influence goes in the same direction in both sexes, the trend to increase anthropometric measures differs in both sexes, with a significant accumulation of abdominal adiposity in girls solely, thus demonstrating the importance of considering SM in nutritional assessment of children and adolescents.
REFERENCES
1. World Health Organization. Obesity: preventing and managing the global epidemic. Geneva, 2000 (Technical Report Series, 894). [ Links ]
2. Pan American Health Organization. Globesity: The crisis of growing proportions. Pers-pec Health Mag. 2002; 7:6-11. [ Links ]
3. Ogden CL, Flegal KM, Carroll MD, et al.. Prevalence and trends in overweight among US children and adolescents, 1999-2000. J Am Med Assoc 2002; 288:1728-1732. [ Links ]
4. Ogden CL, Carroll MD, Curtin LR, et al.. Prevalence of overweight and obesity in United States 1999-2004. J Am Med Assoc 2006; 295:1549-1555. [ Links ]
5. Neutzling MB, Taddei JAAC, Rodrigues EM, et al.. Overweight and obesity in Brazili-an adolescents. Int J Obes 2000; 24:869-874. [ Links ]
6. Veiga GV, Cunha AS, Sichieri R. Trends in overweight among adolescents living in the poorest and richest regions of Brazil. Am J Public Health 2004; 94:1544-1548. [ Links ]
7. Cintra IP, Passos MAS, Fisberg M, et al.. Evolução de duas séries históricas do índice de massa corporal em adolescentes. J Pedatr (Rio J) 2007; 83:157-162. [ Links ]
8. Taylor RW, Falorni A, Jones IE, et al.. Indentifying adolescents with high percentage body fat: a comparison of BMI cutoffs using age and stage of pubertal development compared with BMI cutoffs using age alone. Eur J Clin Nutr 2003;57:764-769. [ Links ]
9. Sievorgel RM, Demerath EW, Schuber C, Remsberg KE, et al.. Puberty and body com-position. Horm Res 2003; 60:36-45. [ Links ]
10. Pereira FN, Oliveira JR, Zölner CC, Gambardella AMD. Body weight perception and associated factors in students. J Human Growth And Development 2013; 23(3): 196-302. [ Links ]
11. Frisch RE, Revelle R. Height and weight at menarche and a hypothesis of critical body weights and adolescent events. Science 1970;169:397-379. [ Links ]
12. Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child 1969; 44:291-303. [ Links ]
13. Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child 1970; 45:13-23. [ Links ]
14. Adair LS, Gordon-Larsen P. Maturational timing and overweight prevalence in US ado-lescent girls. Am J Public Health 2001:91:642-644. [ Links ]
15. Wang Y. Is obesity associated with early sexual maturation? A comparison of the asso-ciation in American boys versus girls. Pediatrics 2002;110:903-910. [ Links ]
16. Ribeiro J, Santos P, Duarte J, et al.. Association between overweight and early sexual maturation in Portuguese boys and girls. Ann Hum Biol 2006; 33:55-63. [ Links ]
17. Lohman TG, Roche AF, Martorell R. Anthropometric standardization reference manual. Illinois: Human Kinetics Books 1988. [ Links ]
18. Conde WL, Monteiro CA. Body mass index cutoff points for evaluation of nutritional status in Brazilian children and adolescents. J Pediatr (Rio J). 2006; 82:266-272. [ Links ]
19. Tanner J. Growth at adolescence. 2nd ed. Oxford: Blackwell Scientific Publications, 1962; 36-39. [ Links ]
20. World Health Organization. Physical status: the use and interpretation of anthropome-try. Geneva, 1995 (Technical Report Series, 854). [ Links ]
21. de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ. 2007;85:660-7. [ Links ]
22. Cole TJ, Green P J. Smoothing reference centile curves: the LMS method and penalized likelihood. Stat Med 1992;11:1305-1319. [ Links ]
23. Statacorp LP. Stata Statistical Software: Release 10.1. College Station, TX: Stata Cor-poration, 2007. [ Links ]
24. Chinn S, Rona RJ. International definitions of overweight and obesity for children: a lasting solution? Ann Hum Biol 2002;29:306-313. [ Links ]
25. Cole TJ, Bellizzi MC, Flegal KM, et al.. Establishing a standard definition for child overweight and obesity worldwide: international survey. Br Med J 2000;320:1-6. [ Links ]
26. Wang Y, Wang JQ. A comparison of international references for the assessment of child and adolescent overweight and obesity in different populations. Eur J Clin Nutr 2002;56:973-982. [ Links ]
27. Duke PM, Litt IF, Gross RT. Adolescents' self-assessment of sexual maturation. Pedia-trics1980; 66:918-920. [ Links ]
28. Saito MI. Maturação sexual: auto-avaliação do adolescente. Pediatr (São Paulo). 1984;6:111-5. [ Links ]
29. Bonat S, Pathomvanich A, Keil MF, et al.. Self-assessment of pubertal stage in over-weight children. Pediatrics 2002;110:743-747. [ Links ]
30. Desmangles JC, Lappe JM, Lipaczewski G, et al.. Accuracy of pubertal Tanner staging self-reporting. J Pediatr Endocrinol Metab 2006;19:213-221. [ Links ]
31. Chan N, Sung RY, Kong AP, et al.. Reliability of pubertal self-assessment in Hong Kong. J Pediatr Child Health 2008; 44:353-358. [ Links ]
32. Stephen MD, Bryant WP, Wilson DP. Self-assessment of sexual maturation in children and adolescents with diabetes mellitus. Endocr Pract 2008; 14:840-845. [ Links ]
33. Papadimitriou A, Pantsiotou S, Douros K, et al.. Timing of pubertal onset in girls: evi-dence for non-Gaussian distribuition. J Clin Endocrinol Metab 2008; 93:4422-4425. [ Links ]
34. Kaplowitz PB, Oberfield SE, Drug and Therapeutics Executive Committees of the Lawson Wilkins Pediatric Endocrine Society. Reexamination of the age limit for defin-ing when puberty is precocious in girls in the United States: implications for evaluation and treatment. Pediatrics 1999; 104:936-941. [ Links ]
35. Pantsiotou S, Papadimitriou A, Douros K, et al.. Maturational tempo differences in rela-tion to the timing of the onset of puberty in girls. Acta Paediatr 2008; 97:217-220. [ Links ]
36. Himes JH, Obarzanek E, Baranowski T, et al.. Early sexual maturation,body composi-tion, and obesity in African-American girls. Obes Res 2004;12:64-72S. [ Links ]
37. Bratberg GH, Nilsen TI, Holmen TL, et al.. Early sexual maturation, central adiposity and subsequent overweight in late adolescence. A four-year follow-up of 1605 adoles-cent Norwegian boys and girls: the Young HUNT study. BMC Public Health 2007; 7:54-60. [ Links ]
38. Chen X, Wang Y. The influence of sexual maturation on blood pressure and body fat-ness in African-American adolescent girls and boys. Am J Hum Biol. 2009; 21:105-112. [ Links ]
39. Slyper AH. The pubertal timing controversy in the USA, and a review of possible caus-ative factors for the advance in timing of onset of puberty. Clinical Endocrinology. 2006;65:1-8. [ Links ]
Manuscript submitted Oct 08 2013
Accepted for publication Feb 22 2014
Corresponding author: gambarde@usp.br










 text in
text in