Services on Demand
article
Indicators
Share
Journal of Human Growth and Development
Print version ISSN 0104-1282On-line version ISSN 2175-3598
J. Hum. Growth Dev. vol.30 no.1 São Paulo Jan./Apr. 2020
http://dx.doi.org/10.7322/jhgd.v30.9962
ORIGINAL ARTICLE
Cardiac autonomic modulation in juvenile idiopathic arthritis with use of biological medication
Modulação autonomica cardíaca na artrite idiopática juvenil com uso de medicação biológica: relato de caso
Patrícia Merly MartinelliI, II, III; Ana Carolina Gonçalves de AbreuI; José Rener Cordeiro da SilvaIII; Asami Takahara VasconcelosIII; Adilson MonteiroI, IV; Valdelias Xavier PereiraI; Rodrigo Daminello RaimundoI
ILaboratório de Delineamento de Estudos e Escrita do Centro Universitário Saúde ABC, Santo André, São Paulo, Brasil
IICentro Universitário UniDomBosco, Curitiba - (PR), Brasil
IIILaboratório de Delineamento de Estudos e Escrita Científica do Centro Universitário UNINORTE - Rio Branco (AC), Brasil
IVUniversidade Federal do Mato Grosso (UFMT) - Rondonópolis (MT), Brasil
ABSTRACT
INTRODUCTION: The introduction of biological medication in Juvenile Idiopathic Arthritis (JIA) proposes better therapeutic results with decreased pain and inflammation and consequent reduction in joint damage. The autonomic state can be a predictor for verifying the response to immunomodulation therapy. Thus, measuring heart rate variability can express autonomous behavior and possibly accompany the response to therapy through the expression of the inflammatory condition
ObjectiveAnalysis of heart rate variability in a child with Juvenile Idiopathic Arthritis using the anti-Tumor Necrosis Factor.
METHODS: This is a clinical case report of an 8-year-old male with a diagnosis of JIA - oligoarticular form, using etanercept, admitted to Clínica Escola de Fisioterapia UNINORTE, Acre, Brazil in 2017. We analyzed laboratory and imaging tests, kinetic-functional evaluation, examination of cardiac autonomic modulation and physiotherapeutic treatment for analgesic, anti-inflammatory purposes, gaining flexibility, strength and postural re-education, according to CARE guidelines, case report guidelines
RESULTS: After medication administration, there was a decrease in pain and normalization of serum creatinine (0.50 mg / dL) and CRP (less than 6 mg / dL) and an increase in erythrocyte sedimentation rate (17 mm3). In the examination of heart rate variability, the linear indices in the time domain showed a predominance of parasympathetic activity (RMSSD: 35ms), with decreased sympathetic control measured through the frequency domain (LF: 27.1 un). However, non-linear methods showed low variability with little dispersion of RR intervals
CONCLUSION: In the present report, the linear indices showed parasympathetic predominance and in the non-linear analysis a low heart rate variability with abnormal and insufficient adaptation of the autonomic nervous system in a child with juvenile idiopathic arthritis using biological medication
Keywords: juvenile arthritis, inflammation, etanercept, autonomic nervous system, heart rate variability.
Authors summary
Why was this study done?
To describe cardiac autonomic behavior in a child using biological medication in order to understand the neurological influence of autoimmunity in this type of case.
What did the researchers do and find?
The authors described a case report of an 8-year-old male child diagnosed with JIA - oligoarticular form, using etanercept (biological medication) through laboratory and imaging exams, kinetic-functional evaluation and cardiac autonomic modulation examination.
What do these findings mean?
The description of childhood autonomic behavior of a boy undergoing biological drug treatment alerts the scientific community to a low-cost measurement test and mild discomfort such as HRV, which is an effective follow-up method.
INTRODUCTION
Juvenile idiopathic arthritis (JIA) consists of a rheumatic disease characterized by chronic inflammation of the joints, lasting at least 6 weeks, unknown etiology, and onset before 16 years old1. It affects approximately 1 in every 1000 children in the world, with great variation of the prevalence by the different existing subtypes2. Rigante et al.3 describe a frequency of 16 to 150 cases per 100,000 children.
The treatment of JIA has taken place in the last 10 years with the introduction of biological or immunobiological therapies aiming to inhibit Tumor Necrosis Factor alpha (TNFα) and cytosine. This change brought improvements in therapeutic outcomes and quality of life. However, it restrains a discussion on the safety of the use of biological drugs4,5.
The autonomic system is related to the inflammatory disease's expression, consisting of a therapeutic or cofactor target that influences the treatment. This can be seen by examining the HRV6,7. Autonomic status may be a predictor to verify response to anti-TNF therapy4,6.
Thus, understanding the neurological influence of autoimmunity comprises current and important thematic for the outcome of this condition4,8. In this study, we attempted to describe the HRV in a child with JIA using the anti-tumor necrosis factor (etanercept).
METHODS
This is a case report of an 8 year-old, male, with a diagnosis of JIA - oligoarticular form, using etanercept, admitted to the Physiotherapy's Clinic School UNINORTE, Acre, Brazil, in 2017. Laboratory and imaging exams were evaluated and kinetic-functional evaluation, cardiac autonomic modulation examination and physiotherapeutic treatment with analgesic, anti-inflammatory, flexibility gain, strength and postural reeducation were performed. The manuscript was approved by the Ethics and Research Committee under the number of opinion 2,766,017/2018 and followed the CARE guidelines, case report guidelines.
CASE REPORT
Patient's identification: T. A. F., 8 years old, male, dark skinned, public student of the basic level, native from Rio Branco - AC/Brasil, living with parents and two siblings.
Main complaint and duration: Forwarded to the Physiotherapy Service of the Physiotherapy's Clinic School UNINORTE in April 2017, with pain complaints in lower limbs (LMI) and claudication.
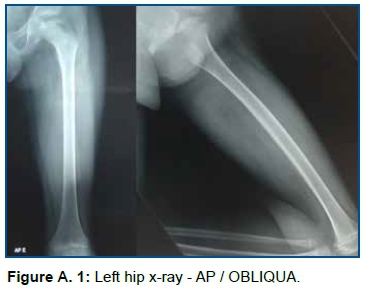
Previous History of the present disease: Clinical diagnosis of Juvenile Idiopathic Arthritis, oligoarticular form. The patient presented tonsillitis and sporadic colds, pain in the lower limbs (hip, knee and ankle) and lumbosacral spine, left hip discrepancy (1 cm) with antalgetic gait. The previous history reveals the onset of pain at the age of four, in 2015, followed by pain and inflammation in the joints of the hip, knee, ankle, lumbar and sacral spine, between acute and remission status, Nuclear Magnetic Resonance (NMR) of the left hip (2015.08.08) presented avascular necrosis of the femoral head. The clinical diagnosis of JIA was confirmed at the age of seven, with hemosedimentation rate:14 mm in the first hour, reference values (RV): 0 to 20 in the first hour and normal C - reactive protein (less than 6 mg/dL, RV: negative - lower than 6.0 mg/dL) and decreased serum creatinine (0.43 mg/dL, RV: 0.70 to 1.20 mg/dL) with a positive response to drug treatment with metotroxane (10 mg) and prednisolone 5 ml 12/12h (1mg/ml). In August 2016, there was an increase in C-reactive protein (0.50 mg/dL, RV: 0 to 5 mm3) and hemosedimentation rate (11 mm3 in the first hour, RV: 0 to 5 mm3), serum creatinine (0.49 mg/dL). X-ray (2016.05.06) showed impaction, sclerosis, irregularity and deformity of the femoral head's epiphysis (Figure A.1). The NMR examination, on 2017.08.06, identified avascular necrosis of the femoral head, characterized by fragmentation, collapse and sclerosis of the femur's proximal epiphyseal ossification center, associated with moderate stroke/synovial thickening in the hip (Figure A.2) .
Interrogation about devices: deny.
Personal and family history: deny.
Clinical Findings
General and specific clinical examination: in August/2016 presented good general condition, ruddy, hydrated, afebrile (axillary temperature: 36.6ºC), normal heart rate variability: 76 beats per minute (bpm), normotensive (100/60 mmHg), eupneic (respiratory rate 23 irp/m), heart and lung auscultation without relevant findings, weight: 28,400 kg, height: 124 cm, normal body mass index (BMI): 18.21 kg/m2. Distended and flaccid abdomen, with no pain complaint in palpation, positive Fabere test to the left, pain while performing the test and a lot of resistance.
Kinetic-functional evaluation: presented a pain complaint during hip and left knee movements and to prolonged orthostatism, with irradiation to the anterior region of the left lower limb. Decreased muscle strength (MS), Impaired flexibility in left hip flexion (100°) and preserved reflexes. Asymmetry in lower limbs with right-sided scoliosis, lumbar hyperlordosis, abdominal protrusion, bilateral internal femoral rotation, plantar arch collapse, with global static postural alteration (Figure A.3).
Diagnostic: Juvenile idiopathic arthritis - oligoarticular.
Prognostic: With appropriate treatment, joint prognosis is often good in patients whose disease is limited to a few joints. With the introduction of the biological agent we sought remission or low disease activity and prevention of irreversible joint damage.
Therapeutic Intervention
Medical conduct: Laboratory and complementary exams were requested and an active phase of the disease was verified with refractory response to conventional therapy. The rheumatologist prescribed etanercept (25 mg) through monthly assisted infusions (August/2016).
Physiotherapeutic conduct: minimization of the pain and inflammation, maintenance of muscle trofism and range of motion.
Adverse and unforeseen events: the patient presented recurrent pharyngotonsillitis.
Laboratory tests: In February 2017, serum creatinine values of 0.50 mg/dL (RV: <0.50) and hemosedimentation rate of 17.00 mm3 in the 1st hour (RV: 0 to 5 mm3) and C-reactive protein (less than 6 mg/dL, VR: negative - less than 6.0 mg/dL).
Complementary examinations
In February/2017, the HRV test was performed by the Polar Rs800cx heart rate monitors, with beat-to-beat recording, and only those series with more than 95% of sinus beats were used for analysis. The capture was subjected to digital filtering in Polar Precision Performance SW software (version 4.01.029) and Kubios9 software. HRV was analyzed in time and frequency domains.
When comparing the linear indexes, time domain, with findings in the literature of healthy children, in the same age group, an increase (35.8 ms) in the RMSSD is observed (square root of the square mean of the differences between successive normal RR intervals, found from the analysis of adjacent RR intervals, in a time interval), which expresses the behavior of the parasympathetic activity. In the frequency domain it is observed that the variable low frequency (LF-0.04-0.15) was decreased (27.1 un), this reveals the sympathetic and parasympathetic behavior through the baroreflex activity, with sympathetic predominance. The high frequency peak (HF-0.15-0.4 Hz) representing the parasympathetic (vagal) activity, influenced by breathing, was elevated (78.2 un). Thus, it was observed a predominance of the parasympathetic ANS on the cardiac autonomic modulation with HRV increase both in the frequency domain and in the time domain.
DISCUSSION
The influence of the autonomic nervous system on the inflammatory disease's response, through it's interaction with the immune system, has been the aim of researches4,5. Studies show that autoimmune disease can affect autonomic homeostasis6,8,10. Research performed in patients with Sjogren's Syndrome showed an increase in the parasympathetic response, with a reduction in LF and an increase in HF, corroborating with the findings of this report11.
Holman et al.4 described the HRV as a predictor exam of response to anti-TNF therapy in patients with inflammatory atritis and emphasizes the importance of autonomic influence on the expression of autoimmune diseases. Boettger et al.12 verified changes in rats' HRV and observed that initially there was a predominance of sympathetic ANS, followed by a reduction of this tone after the administration of etanercept. The authors suggest that the neutralization of TNF alpha reduces sympathetic tone due to the reduction of the peripheral inflammatory response caused by the medication.
A prospective analysis of the HRV in psoriatic patients demonstrated a significant decrease in sympathetic oscillatory (LF%) and low/high frequency (LF/HF) components after treatment with etanercept. Thus, it was evidenced that the use of the medication can affect the cardiac autonomic modulation and reduce the cardiovascular risk13.
The use of anti tumor necrosis factors has shown a decrease in inflammatory response, with delayed bone degeneration and improvement in patients' quality of life4,12.
The novelty of the study is the description of the autonomic behavior in the boy's childhood in biological drug treatment, alerting the scientific community to a low-cost measurement and mild discomfort examination such as HRV, consisting of an effective method of follow-up.
The case report is related in the scientific literature as the type of initial study design to develop new possibilities of study design, being limited in bringing results of causality and reproducibility. Perceptions of the patient involved were not reported because they were not appropriate in this case report.
Studies have sought to describe the mechanisms of neuroendocrine regulation and it's influence on inflammatory diseases4,8,10,14-16. The investigations are based on the effect of inflammatory cytokines on sympathetic activity and feedback cycles; existence of correlation between inflammation and HRV markers; an interaction of sympathetic predominance with inflammatory processes or even TNF itself as a direct depressant of HRV, causing an attenuation of beta adrenergic signaling4,8,17.
In this case's report a parasympathetic predominance and insufficient adaptation of the autonomic nervous system was observed. Thus, there is a need for greater discussion about the topic for understanding and standardization of autonomic behavior in immunomodulators18.
CONCLUSION
In the present report linear indexes presented a parasympathetic predominance with an increase in HRV, evidencing a good adaptation of the autonomic modulation before the treatment.
Acknowledgement
To Northern Educational Union (UNINORTE/AC), for collaborating and encouraging scientific awakening. To the researchers Isabel Cristina Esposito Sorpreso, Maithe Blaya Leite, Natália da Silva Freitas Marques, Franciely Gomes Gonçalves, Rosicley Souza da Silva, Candido Ferreira Rodrigues Neto, Alex Rey Norberto and Valdelias Xavier Pereira for their participation and motivating partnership. To the rheumatologist who accompanies the case, Adriana de Oliveira Marinho, who brilliantly conducts the clinical treatment and collaborated promptly with the report. To the patient and his family, who thought about their fellow citizens when they accepted to participate in the study.
REFERENCES
1.Blazina S, Markelj G, Avramovic ZM, Toplak N, Avcin T. Management of Juvenile Idiopathic Arthritis: a clinical guide. Paediatr Drugs. 2016;18(6):397-412. DOI: https://dx.doi.org/10.1007/s40272-016-0186-0 [ Links ]
2.Huang JL. New advances in juvenile idiopathic arthritis. Chang Gung Med J. 2012;35(1):1-14. DOI: http://dx.doi.org/10.1136/adc.2009.170860 [ Links ]
3.Rigante D, Bosco A, Esposito S. The Etiology of Juvenile Idiopathic Arthritis. Clin Rev Allergy Immunol. 2015;49(2):253-61. DOI: https://dx.doi.org/10.1007/s12016-014-8460-9 [ Links ]
4.Holman AJ, Ng E. Heart rate variability predicts anti-tumor necrosis factor therapy response for inflammatory arthritis. Auton Neurosci. 2008;143(1-2):58-67. DOI: https://doi.org/10.1016/j.autneu.2008.05.005 [ Links ]
5.Mota LMH, Cruz BA, Brenol CV, Polak PF, Pinheiro GRC, Laurindo IMM, et al. Segurança do uso de terapias biológicas para o tratamento de artrite reumatoide e espondiloartrites. Rev Bras Reumatol. 2015; 55(3):281-309. DOI: http://dx.doi.org/10.1016/j.rbr.2014.06.006 [ Links ]
6.Malave HA, Taylor AA, Nattama J, Deswal A, Mann DL. Circulating levels of tumor necrosis factor correlate with indexes of depressed heart rate variability: a study in patients with mild-to-moderate heart failure. Chest. 2003;123(3):716-24. DOI: https://doi.org/10.1378/chest.123.3.716 [ Links ]
7.Abreu LC. Variabilidade da frequência cardíaca como marcador funcional do desenvolvimento. J Hum Growth Dev. 2012;22(3):279-82. DOI: http://dx.doi.org/10.7322/jhgd.46712 [ Links ]
8.Straub RH, Bijlsma JW, Masi A, Cutolo M. Role of neuroendocrine and neuroimmune mechanisms in chronic inflammatory rheumatic diseases--the 10-year update. Semin Arthritis Rheum. 2013;43(3):392-404. DOI: https://doi.org/10.1016/j.semarthrit.2013.04.008 [ Links ]
9.Raimundo RD, Abreu LC, Adami F, Vanderlei FM, Carvalho TD, Moreno IL, et al. Heart rate variability in stroke patients submitted to an acute bout of aerobic exercise. Transl Stroke Res. 2013;4(5):488-99. DOI: https://doi.org/10.1007/s12975-013-0263-4 [ Links ]
10.Adlan AM, van Zanten JJCSV, Lip GYH, Paton JFR, Kitas GD, Fisher JP. Cardiovascular autonomic regulation, inflammation and pain in rheumatoid arthritis. Auton Neurosci. 2017;208:137-45. DOI: https://doi.org/10.1016/j.autneu.2017.09.003 [ Links ]
11.Tumiati B, Perazzoli F, Negro A, Pantaleoni M, Regolisti G. Heart rate variability in patients with Sjögren's syndrome. Clin Rheumatol. 2000;19(6):477-80. DOI: https://doi.org/10.1007/PL00011180 [ Links ]
12.Boettger MK, Weber K, Grossmann D, Gajda M, Bauer R, Bär KJ, et al. Spinal tumor necrosis factor alpha neutralization reduces peripheral inflammation and hyperalgesia and suppresses autonomic responses in experimental arthritis: a role for spinal tumor necrosis factor alpha during induction and maintenance of peripheral inflammation. Arthritis Rheum. 2010;62(5):1308-18. DOI: https://doi.org/10.1002/art.27380 [ Links ]
13.Potenza C, Raimondi G, Pampena R, Proietti I, Viola G, Bernardini N, et al. Cardiovascular risk evaluation through heart rate variability analysis in psoriatic patients before and after 24 weeks of etanercept therapy: Prospective study. J Int Med Res. 2016; 44(1 suppl):43-7. DOI: https://doi.org/10.1177/0300060515593242 [ Links ]
14.Davies R, Gaynor D, Hyrich KL, Pain CE. Efficacy of biologic therapy across individual juvenile idiopathic arthritis subtypes: A systematic review. Semin Arthritis Rheum. 2017;46(5):584-93. DOI: https://doi.org/10.1016/j.semarthrit.2016.10.008 [ Links ]
15.Ricci-Vitor AL, Rossi FE, Hirai PMH, Silva NT, Vanderlei FM, Haddad MI, et al. Effects of a multidisciplinary program on autonomic modulation in overweight or obese children and adolescents. J Hum Growth Dev. 2016;26(1): 154-61. DOI: http://dx.doi.org/10.7322/jhgd.119257 [ Links ]
16.Alves SAA, Oliveira MLB. Sociocultural aspects of health and disease and their pragmatic impact. J Hum Growth Dev. 2018;28(2):183-8. DOI: http://dx.doi.org/10.7322/jhgd.147236 [ Links ]
17.Lanza GA, Sgueglia GA, Cianflone D, Rebuzzi AG, Angeloni G, Sestito A, et al. Relation of heart rate variability to serum levels of C-reactive protein in patients with unstable angina pectoris. Am J Cardiol. 2006;97(12):1702-6. https://doi.org/10.1016/j.amjcard.2006.01.029 [ Links ]
18.Abreu LC, Pereira VX, Silva RPM, Macedo Jr H, Bezerra IMP. The right to scientific information: one of the main elements of the unified health system. J Hum Growth Dev. 2017;27(3):258-261. DOI: http://dx.doi.org/10.7322/jhgd.141485 [ Links ]
 Correspondence:
Correspondence:
martinelli_patricia@hotmail.com
Manuscript received: November 2019
Manuscript accepted: February 2020
Version of record online: March 2020










 text in
text in