Servicios Personalizados
Articulo
Indicadores
Compartir
Journal of Human Growth and Development
versión impresa ISSN 0104-1282versión On-line ISSN 2175-3598
J. Hum. Growth Dev. vol.31 no.1 Marília ene./abr. 2021
http://dx.doi.org/10.36311/jhgd.v31.11209
ORIGINAL ARTICLE
DOI: 10.36311/jhgd.v31.11209
Prevalence of obesity, high blood pressure, dyslipidemia and their associated factors in children and adolescents in a municipality in the Brazilian Amazon region
Juliana de Souza Almeida Aranha CamargoI, II; Tallita Beatriz de Oliveira ZamarchiI; Antônio Alcirley da Silva BalieiroIII, IV; Felipe Arley Costa PessoaI; Luís Marcelo Aranha CamargoI, II, V, VI, VII
IOswaldo Cruz Foundation Leônidas and Maria Deane Institute, Manaus, Amazonas, Brazil (Post-Graduation Student)
IIDepartment of Medicine, Afya/São Lucas University, Porto Velho, Rondonia, Brazil
IIIResearch Support Team, Oswaldo Cruz Foundation Leônidas and Maria Deane Institute, Manaus, Amazonas, Brazil
IVLaboratory of Ecology of Communicable Diseases in the Amazon, Oswaldo Cruz Foundation Leônidas and Maria Deane Institute, Manaus, Amazonas, Brazil
VCoordinator of the Biomedical Science Institute 5, University of São Paulo, Monte Negro, Rondonia
VINational Institute of Science & Technology, Porto Velho, Rondonia, Brazil
VIIDepartment of Medicine,Tropical Medicine Center, Porto Velho, Rondonia, Brazil
ABSTRACT
INTRODUCTION: The incidence of chronic noncommunicable disease (CND) are rocketting over the world, including in young adults. The WHO estimates that more than half of the deaths in the world, even in underdeveloped countries, are caused by CND.
OBJECTIVES: The study aimed to estimate the prevalence of obesity, high blood pressure (HBP) and dyslipidemia and its associated factors.
METHODS: The authors carried out a cross-sectional study of 1,431 schools in the public-school system of Monte, Brazilian Western Amazon, with children and adolescents aged 6 to 15 years. A random sampling of 496 individuals was carried out. The OpenEpi platform was used to calculate the sample size, considering p<0.05 and a presumed prevalence of CND of 50%. The authors applied a clinical-epidemiological questionnaire, made anthropometric measurements and laboratory tests. Diagnostic parameters recommended by the recent guidelines of the Ministry of Health in Brazil were used.
RESULTS: Prevalence of CND was: Obesity 11.8%, HBP of 6.7% and dyslipidemia of 25.4%. After multivariate log-binomial analysis of the dependent variables, the statistically significant risk factors were overweight 18.4%, sedentary lifestyle 32.2%, family history of cardiovascular disease 23.4%, family history of HBP 84.2%, family dyslipidemia 55.8%, family obesity 38.7% and family chronic renal disease 40.6%.
CONCLUSION: The findings pointed out to a context with a relatively high prevalence of CND, as well as their associated factors. Intervention measures such as health education, food education, stimulation of physical exercise, better school feeding and an improvement of the public health system are needed to mitigate the occurrence of CND.
Keywords: Brazilian Amazon, Chronic Noncommunicable Disease, Students.
Authors summary
Why was this study done?
As two previous studies, carried out between 2014 and 2015 in the municipality of Monte Negro pointed for extremely high prevalence's of Chronic Noncommunicable Disease in the elderly. As so, the authors supposed that children and adolescents already had CND and/or risk factors to justify so elevated prevalence´s in the elderly.
What did the researchers do and find?
The authors randomly examined 496 school children/adolescents out of 1,431 individuals and screened for CND and their risk factors. The authors found a high prevalence of CND and a high prevalence of risk factors among the examined population.
What do these findings mean?
It means that children/adolescents are already having CND and exposed to their factor risks, fact that justifies the elevated prevalence of CND in the elderly. Such results point for the weakness of the local public health system, unable to deal with such relevant problem.
INTRODUCTION
Chronic Noncommunicable Disease (CND) is responsible for 63% of the 56.5 million deaths annually worldwide and is the leading cause of death and disability in the world, with obesity, diabetes mellitus type 2 (DM2), poor diet, smoking, high cholesterol, sedentary lifestyle, hypertension and alcohol consumption as the main risk factors1. A recent study, indicates that ischemic heart disease, stroke and DM2 account for more than 25% of the Days of Life Lost and Lived with Disability (DALY) in older than 49 years in the world in 20192. Estimates consider that worldwide one in 20 children is affected by at least one CND3.
Diabetes Mellitus 2 is considered a global health problem, with an estimated prevalence of 4.7% in 1980 and 8.5% in 2014 for the adult population (WHO), with an expected annual increase of 2.2% from 2010 to 20304, 5.
Until the mid-1940s, Brazil's epidemiological profile was characterized by the prevalence of high birth and mortality rates6. Causes related to infectious and parasitic diseases, malnutrition, and reproductive health problems, that historically affected infant and under-five mortality, have lost their predominance, being replaced by CND and external causes6, 7.
In Brazil, DM2 is estimated to account for a DALY of 25.96-2778.69/100,000 in men and 48.18- 2123.47 /100,000 for women in 2015. The DM2 increase HBP been of the order of 35% since 1990, while for the other CND it was 12.7%8.
According to data recorded by the National Health Survey (NHS-2013) 6.2% of the Brazilian population reported a diagnosis of diabetes. In the northern region, 4.3% and 7.1% in the southeastern region, had the highest numbers of people diagnosed with DM2. From that, the indexes show that DM2 increased according to age, being 0.6% in age groups from 18 to 29 years, 5% between 30 and 59 years, 14.5% between 60 and 64 years and 19.9% between 65 and 74 years. It also points to the growth in the number of overweight and obese children and adolescents9.
In 2009, 2012 and 2015, the National Survey of School Health (2016) was conducted. This survey revealed that food quality in schools was poor and student´s physical exercise was restricted to school. Sedentary lifestyle and inadequate nutrition are strong allies for the establishment of the obesity process, which in turn is causally related to the emergence of CND and its risk factors9.
The prevalence of dyslipidemia among children and adolescents in the world ranges from 2.9 to 33% when cholesterol above 200mg/dL is considered.3 In the United States, one study reported that 1 in 5 children between 8 and 17 years of age have some lipid disorder10.
In Brazil, the prevalence of childhood dyslipidemia ranges from 3.1% to 46.5%11. Although mortality from hypertensive heart disease HBP decreased by about 19%, it is responsible for 31,999 deaths per year in the 15-49 age group12. The prevalence of HBP in the pediatric population, when the 90th percentile or higher is considered, varies between 3.5% and 19.2%13.
In Brazil, in a study carried out in the Brazilian capitals, for example, HBP in children and adolescents HBP a prevalence of between 8.4% and 12.5%, being the highest in the southern region15. There is a lack of data on the population of the Amazon, especially in the riverine areas and smaller cities. Other study, carried out in Monte Negro (Brazil), in 2014, pointed for very high prevalence's of CND in the elderly a and a shift in the mortality profile in the last 10 years to a higher mortality profile caused by CND17.
This study aims to assess the prevalence of some CND and its associated factors in children and adolescents. It also aims and to collaborate in the development of intervention strategies to reduce the risk factors for its occurrence.
METHODS
Cross-sectional study. For the present study, a prevalence of 50% CND (unknown prevalence) was estimated, with a hypothetical sample loss of 30%, and a randomized stratified proportional school sample of 496 individuals between 6 and 15 years of age from a universe of 1,431 children enrolled in the three existing urban public schools in Monte Negro was used. The OpenEpi platform was used to calculate the sample.
The study area is located in the western Brazilian Amazon, 250 km northwest of Porto Velho (state capitol), in the municipality of Monte Negro, with a latitude of 10°15' 6" south, and longitude of 63° 17' 14" west. A population of 15,695 was estimated for 2018. Data were collected from August to November 20.
Individuals over 5 years of age and under 16 years of age who had agreed to participate in the study, who had the consent of those responsible and have fasted for at least 8 hours were included in the study.
For everyone, parental consent, or a close relative, fulfilled an informed consent form to participate in the study under the ethical national committee CAAE number 49569515.8.0000.0013.
A clinical-epidemiological questionnaire was applied to children/adolescents aged 6 to 15 years and parents. At the same time, a questionnaire was also applied to evaluate sleeping time, consumption of soft drinks and breakfast at home. Individuals were asked the time they went to bed and the time they woke up, whether they consumed soft drinks and how often they consumed them during the week, and whether they were in the habit of having coffee at home.
Ten milliliters of blood were collected by a venous puncture after antisepsis, (4 mL in fluoridated tube and 6 mL in a dry tube) for biochemical analysis of fasting glycemia and lipid levels. The fasting glycemia and lipid profile tests were performed using Labtest® colorimetric kits in LABMAX PLENNO® (Labtest Diagnóstica S.A - Brazil). For the diagnosis of glucose intolerance, results were considered >99mg/dL and <126 mg/dL and for DM2 values >125 mg/dL. To characterize dyslipidemia, triglyceride levels >99 mg/dL for children <10 years and triglycerides >129 mg/dL for children >10 years and/or simultaneously altered LDL/Triglycerides characterizing mixed dyslipidemia were considered. The value of HDL was considered low, regardless of gender, when less than 40 mg/dL18.
The anthropometric indices were obtained from basic anthropometric information (weight, gender, age, height). The children were weighed using a digital scale with a capacity of 150 kg and accuracy of 50g and were measured standing and barefoot using a fixed tape measure. They were assessed BMI (Body Mass Index by Age), using a calculation based on the WHO Anthroplus software, Version January 2011). Overweight was considered when the BMI score was between +1 and +2 and obesity >+2.
Blood pressure (BP) was measured according to the VII Guideline of the Brazilian Society of Cardiology (BSC), where the child was at rest, seated, for at least 5 minutes before the first BP measurement was obtained. Three measurements were performed on the right arm, where the first was discarded. The BP assessment was carried out by two different professionals, trained and blind, and the kappa index was calculated by the proportion of hits between the two professionals. The method of choice was auscultatory and the sphygmomanometer was aneroid suitable for the diameter of the arm. The American Pediatric Hypertension pressure calculator of the American Society of Pediatrics was used, which defines blood pressure limits according to gender, age and height percentile.
The International Physical Activity Questionnaire (IPAQ3), version 8, short form, the usual week for an assessment of students' level of physical activity was applied individually.4 Considering the following categories: Non-sedentary, irregularly active and sedentary.
The data were stored in the Excel® spreadsheet. The statistical software "R" was used in version 3.6.0 (R Development Core Team, 2019), using the Rstudio version 1.1.4, with packages tidyverse, epiDisplay, hnp, logbin and sjPlot. First, the prevalence was estimated with a 95% confidence interval. In a second step, a statistical association was applied between all explanatory variables one by one, using the Fisher´s test. It was stated that the variables with p<0,25 should be submitted to a multivariate log-binomial.
Three adjustments of the log-binomial model were made for the dependent variables: Dyslipidemia, HBP and Obesity (outcomes) considering the explanatory variables selected in the study. The model was chosen because it provided the prevalence ratio (PR) as an association measure, more appropriate for cross-sectional studies19, 20. The next step was to use the backward selection strategy, in which all pre-selected explanatory variables were placed in the model and removed one by one based on significance statistics with p<0.05. The models chosen for each outcome were plotted on Forest plot graphs.
RESULTS
We found a homogeneity in the distribution of the sample by gender, approximately 70% lived in urban areas and 98% were from Rondonia as shown in Table 1.
To evaluate the accuracy among the professionals responsible for measuring anthropometric data, the kappa index was performed between two professionals and the kappa index of 80% was obtained, showing good reliability of the method.
The study found the high prevalence of CND and its associated factors, drawing attention to obesity and dyslipidemia. Tables 2 to 3
Regarding the median time of use of mobile phone/television it was observed 3 hours/day for both genders. We found one case of Diabetes Mellitus type 1, no cases of DM2 and 26 cases of glucose intolerance with no difference by gender.
After univariate analysis using Fisher's chi-square test, the following associated factors with p<0.25 were selected for the outcomes below:
HBP: Obesity (p<0.001), Overweight (p=0.059), History of Familial DM2 (p=0.106), History of Familial Dyslipidemia (p=0.099) and History of Familial Cardiovascular Disease (p=0.157).
Dyslipidemia: History of Familial DM2 (p=0.202) and Sedentary Lifestyle (p=0.118).
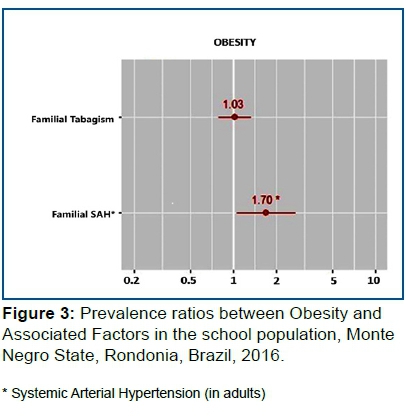
Obesity: Family Income (p<0.094), Familial History of SAH (p<0.001), Familial History of Obesity (p<0.127) and Familial History of Tabagism (p<0.021).
The associated factors selected above (p<0.25), in turn, were submitted to multivariate log-binomial analysis and the following associations were found with p<0.05 (Figure 1):
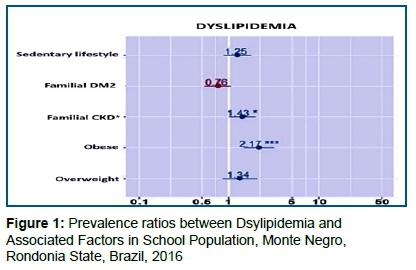
Figure 1 Indicates that for BMI (which we have three categories, "Normal" being the reference category) using the combination "Obese" versus "Normal" against the individual having dyslipidemia. The risk of dyslipidemia was 2.19 higher for obese (p < 0.001) than for an individual with a BMI that is considered normal. On the other hand, the overweight individual (p=0.310) does not present a risk that can be considered different from an individual with a normal BMI.
Following the previous reasoning, the individual with a history of Chronic Familial Kidney Disease presented a risk of dyslipidemia 1.47 higher than individuals with no history of Chronic Familial Kidney Disease (p=0.018).
Figure 2 indicated that obese individuals have a higher risk of HBP than non-obese individuals. In other words, the risk of HBP is 7.96 higher in obese individuals (p<0.001) than in individuals with BMI considered normal. As well, the risk of HBP is 2.69 higher in those with a history of Familial Dyslipidemia than in those with no history of Familial Dyslipidemia (p = 0.024).
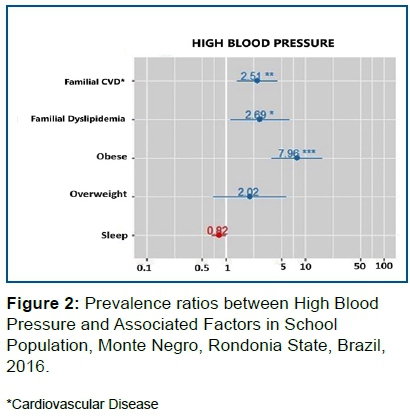
The risk of HBP is 2.51 higher in individuals with a history of Family Cardiovascular Diseases than in individuals without Family Cardiovascular Diseases. (p = 0,002)
In Figure 3, the risk of being Obese is 1.7 higher in those with a history of Familial SAH (p=0.028) than in individuals without Familial HBP. Familial smoking history was not associated with the obesity outcome.
DISCUSSION
Previous studies describe the profile of the population of Monte Negro as being of low schooling, low income, mixed and with a mixed morbidity and mortality profile where infectious diseases, CND, and violence prevail17.
The elderly has a high prevalence of CND and the CND proportional mortality coefficient exceeds 50%17. This finding, in particular, HBP led to the questioning of why the proportion of CND in the elderly population is so high. It was, therefore, imagined that this fact could be generated, in a non-exclusive way, by a high set of associated factors in children, who develop and age in an environment with an inefficient health system from the point of view of mitigating the evolution of CND, allied to the physiological process of aging, genetic load, and influence of environmental factors. This study intends to investigate the first of these components: the health of the child, the elderly in the future.
Socio-economic and demographic data
Data was not explored in this study, but observed by researchers, who even had children attending public schools, point to an environment of little practice of regular physical exercise, being replaced by the most diverse playful activities that do not require physical activity (dominoes, game of checkers, games on the mobile phone, etc.). High schools have a deteriorated multi-sport court where once a year "Olympics" take place between classes (personal information).
In school meals, foods rich in carbohydrate (biscuits, sweetened artificial juices, pasta, margarine) prevail without the adequate supply of fruits or food rich in fiber (personal information).
These two factors can contribute synergistically to the high prevalence of obesity in the population studied.
HBP, Dyslipidemia and Obesity
In this study, HBP presented an overall prevalence of 6.8% (95% CI 4.9 - 9.4), with no significant difference between genders. In South Africa, there was a prevalence of HBP in children ranging from 2.3 to 5.9%21. In the central-western United States, the study showed a prevalence of 20% of HBP, where 60% of patients diagnosed with HBP had no intervention22.
The findings of this study are close but lower than those found in the ERICA study, which points to prevalence in adolescents between 8.4 and 12.5% in Brazil, being higher in the southern region15. A study conducted between 1990 and 1991 in Bento Gonçalves (south of Brazil) showed a prevalence of HBP of 8.2%23. More recent data indicate that the numbers of children and adolescents with HBP have been growing in the southern region of the country with prevalence ranging from 11.3 to 17.6%24, 25, 26.
When studying the association of HBP with associated factors obesity, familial history of dyslipidemia and cardiovascular disease, it was observed that these factors significantly increase the risk. There is a well-known association between the association of obesity and HBP27, 28, 29. The increased risk of HBP with a family history of CND (dyslipidemia and cardiovascular disease) reinforces the important genetic component in the determination of this condition as well as the influence of family habits (sedentary lifestyle, inadequate diet and others)27, 30.
Obesity, in this study, showed an overall prevalence of 13.5%, higher than that found by Bloch15 which was 6.6% in adolescents in the northern metropolitan regions. Dyslipidemia, in turn, had a prevalence of 22.6% (95%CI 19,1-26,5), while 6.6% (95%CI 4,7-9,1) had hypertriglyceridemia. Both showed no significant difference between genders. The results found are lower than in more recent related studies, except for that of Bloch15, whose study was limited to adolescents and sampling by convenience.
A study conducted on school children in the interior of São Paulo found a prevalence of obesity of 49.2%, 17.8% of metabolic syndrome, 54.6% of hypercholesterolemia and 42.3% of hypertriglyceridemia, alerting to the high cardiovascular risk of these children in the future31.
According to Sapunar32, in a study of Chilean children in the same age group as this study, a 38% prevalence of dyslipidemia was found, also without correlation with gender. The authors' related dyslipidemia with obesity (54% of people with dyslipidemia), a fact also found in this study. They often observed the relationship between dyslipidemia with increased cardiovascular risk and insulin resistance (54% of ones with dyslipidemia).
Finn28 pointed to early ventricular dysfunction, increased insulin resistance (by visceral fat deposition) and HBP, and an increased risk of death and hospitalization for pancreatitis and/or retinal thrombosis. Generally, these individuals present hypertriglyceridemia, potentiating the increase of cardiovascular risk by increased oxidative stress and increased progression of arteriosclerosis.
A study carried out in a university hospital in Rio de Janeiro, Brazil, in individuals aged 12 to 18 years old by Vizentin33, showed a prevalence of dyslipidemia of 35.1%, with no difference between genders. They pointed to a prevalence of 25.1% obesity and a negative relationship between HDL cholesterol levels and obesity and a positive relationship between obesity and hypertriglyceridemia. In this study, the risk of dyslipidemia was 2.19 higher in obese than in individuals with BMI considered normal. On the other hand, the overweight individual (p=0.310) did not present a risk that could be considered different from the individual with normal BMI.
A systematic literature review by Gonçalves34, points to the occurrence of inadequate diet in 3.3 to 82% of school children and associates this factor with anxiety, stress, increased risk of hospitalization, nutritional deficiencies, obesity and consequently, increased cardiovascular risk. A study by Cote29 corroborates these findings. The inadequacy of school diets is apparent in Monte Negro (although not quantified in this study but reported by the authors) and may justify the existence of CND in children and the high prevalence in Monte Negro's elderly16,17.
In this study, the individual with a history of Familial CKD presented a risk of dyslipidemia, 1.47 higher than individuals with no history of Familial CKD (p=0.018). The relationship between dyslipidemia, atherosclerotic process and renal dysfunction is known20. This association found may reflect a predisposition of this population of schoolchildren and ones with dyslipidemia to develop CKD, thus justifying the high prevalence of CKD in elderly people in Monte Negro, according to a study by Vieira17 and Coelho16.
Finn28 point to the fact that children with dyslipidemia show early ventricular dysfunction, increased insulin resistance (by visceral fat deposition), DM2, non-alcoholic hepatic steatosis and HBP, and an increased risk of death and hospitalization for pancreatitis and/or retinal thrombosis. Generally, these individuals present hypertriglyceridemia, potentiating the increase of cardiovascular risk by increased oxidative stress and increased progression of arteriosclerosis.
Kumar27, correlate obesity with the same diseases cited by Finn28, adding a higher prevalence of obstructive night apnea, a factor known to be associated with HBP and increased cardiovascular risk.
Pomerantz35 and Chan36 still associate obesity with musculoskeletal disorders, often disabling. In the current context, several studies indicate the deleterious relationship between obesity and Sars-Cov-2 infection, relating greater lethality and the need for orotracheal intubation, in addition to admission to the Intensive Care Unit37, 38.
As can be seen in this study, although the prevalence of CND and its associated factors are lower than the studies presented, they exist and can justify the data from Vieira17 and Coelho16, which point to the high prevalence of older people with CND and its sequela in the adult population of Monte Negro27.
Obesity, both in this study and in the literature, points as the main factor for the development of other outcomes. Unfortunately, there is no consensus to address this problem, which is multifactorial. The pharmacological intervention of obesity in children and adolescents is controversial. Some authors suggest treatment for adolescents in extreme cases with elevated LDL and cardiovascular risks indicating statins for treatment39. In cases of severe obesity, lipase inhibitors may be indicated in children older than 12 years, but with little effectiveness40. Bariatric surgery, although few studies have been conducted, may be indicated in obesity grade III with a significant reduction in BMI, although often associated with anemia41.
The lower prevalence of CND and their risk factors in this study may be related to children's lifestyles: the lack of public transport services leading students to walk or cycle to school, the absence of "fast food" restaurants, the low family income making it difficult to buy soft drinks (high sodium and sugar content), and the habit of playing in public spaces may partly justify this lower42.
There is a consensus among the authors of the study that the most appropriate intervention is a lifestyle change with the implementation of healthy diets and appropriate physical activities from community, school, and family level interventions. In the Brazilian national context, such a role could be played by the School Health Program and/or Family Health Strategy Teams.
CONCLUSION
The study shows a high prevalence of obesity, HBP, dyslipidemia and their associated factors. This scenario is linked to a weak public health system and a low concern of a healthy lifestyle among the students and the educators.
REFERENCES
1.Alwan A, MacLean DR, Riley LM, d'Espaignet ET, Mathers CD, Stevens GA, et al. Monitoring and surveillance of chronic non-communicable diseases: progress and capacity in high-burden countries. The Lancet. novembro de 2010; 376(9755): 1861-8. [ Links ]
2.Vos T, Lim SS, Abbafati C, Abbas KM, Abbasi M, Abbasifard M, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. The Lancet. outubro de 2020; 396(10258): 1204-22. [ Links ]
3.Pereira PB, Arruda IKG de, Cavalcanti AMT de S, Diniz A da S. Perfil lipídico em escolares de Recife - PE. Arq Bras Cardiol. outubro de 2010;95(5):606-13. [ Links ]
4.Hagströmer M, Oja P, Sjöström M. The International Physical Activity Questionnaire (Ipaq): a study of concurrent and construct validity. Public Health Nutr. setembro de 2006; 9(6): 755-62. [ Links ]
5.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Research and Clinical Practice [internet]. 2010 Jan [cited 2020 oct 20]; 87(1): 4-14. Available from: https://doi.org/10.10/j.diabres.2009.10.007 [ Links ]
6.Instituto Brasileiro de Geografia e Estatística. Tábua completa de mortalidade para o Brasil - 2013. Breve análise da mortalidade nos períodos 2012-2013 e 1980-2013. Rio de Janeiro, DF: IBGE; 2014. Em https://biblioteca.ibge.gov.br/vizualização/periodicos/3097/tcmb_pdf.2013 acessado em julho 2018. [ Links ]
7.Clinical & biomedical research [Internet]. [citado 26 de fevereiro de 2021]. Disponível em: https://seer.ufrgs.br/hcpa [ Links ]
8.Duncan BB, França EB, Passos VM de A, Cousin E, Ishitani LH, Malta DC, et al. The burden of diabetes and hyperglycemia in Brazil and its states: findings from the Global Burden of Disease Study 2015. Rev bras epidemiol. maio de 2017;20(suppl 1):90-101. [ Links ]
9.Instituto Brasileiro de Geografia e Estatística. Pesquisa nacional de saúde do escolar: 2015 / IBGE, Coordenação de População e Indicadores Sociais. - Rio de Janeiro: IBGE, 2016. 132 p. Em https: //biblioteca.ibge.gov.br/visualizacao/livros/liv97870.pdf acessado em julho 2018. [ Links ]
10.Kit BK, Kuklina E, Carroll MD, Ostchega Y, Freedman DS, Ogden CL. Prevalence of and trends in dyslipidemia and blood pressure among us children and adolescents, 1999-2012. JAMA Pediatr. 1o de março de 2015; 169(3): 272. [ Links ]
11.Borges CQ, Silva R de CR, Assis AMO, Pinto E de J, Fiaccone RL, Pinheiro SMC. Fatores associados à anemia em crianças e adolescentes de escolas públicas de Salvador, Bahia, Brasil. Cad Saúde Pública. abril de 2009; 25(4): 877-88. [ Links ]
12.Brant LCC, Nascimento BR, Passos VMA, Duncan BB, Bensenõr IJM, Malta DC, et al. Variações e diferenciais da mortalidade por doença cardiovascular no Brasil e em seus estados, em 1990 e 2015: estimativas do Estudo Carga Global de Doença. Rev bras epidemiol. maio de 2017; 20 (suppl 1): 116-28. [ Links ]
13.Rosner B, Cook NR, Daniels S, Falkner B. Childhood blood pressure trends and risk factors for high blood pressure: the nhanes experience 1988-2008. Hypertension. agosto de 2013; 62(2): 247-54. [ Links ]
14.Freedman DS, Goodman A, Contreras OA, DasMahapatra P, Srinivasan SR, Berenson GS. Secular trends in bmi and blood pressure among children and adolescents: the bogalusa heart study. PEDIATRICS. 1o de julho de 2012; 130(1): e159-66. [ Links ]
15.Bloch KV, Klein CH, Szklo M, Kuschnir MCC, Abreu G de A, Barufaldi LA, et al. ERICA: prevalences of hypertension and obesity in Brazilian adolescents. Rev Saúde Pública [Internet]. 2016 [citado 26 de fevereiro de 2021]; 50(suppl 1). Disponível em: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0034-89102016000200306&lng=en&tlng=en [ Links ]
16.Schroeder H. The new challenge: the aging process in the brazilian amazonia. J Gerontol Geriatr Res [Internet]. 2015 [citado 26 de fevereiro de 2021]; 04(01). Disponível em: http://www.omicsgroup.org/journals/the-new-challenge-the-aging-process-in-the-brazilian-amazonia-2167-7182-1000200.php?aid=41821 [ Links ]
17.Vieira G de D, Basano S de A, Camargo LMA. Transition of the morbidity and mortality profile in a municipality in the interior of the Brazilian Amazon. Rev Soc Bras Med Trop. agosto de 2016; 49(4): 411-7. [ Links ]
18.Yoon JM. Dyslipidemia in children and adolescents: when and how to diagnose and treat? Pediatr Gastroenterol Hepatol Nutr. 2014; 17(2): 85. [ Links ]
19.Barros AJ, Hirakata VN. Alternatives for logistic regression in cross-sectional studies: an empirical comparison of models that directly estimate the prevalence ratio. BMC Med Res Methodol. dezembro de 2003; 3(1): 21. [ Links ]
20.Coutinho LMS, Scazufca M, Menezes PR. Methods for estimating prevalence ratios in cross-sectional studies. Rev Saude Publica. dezembro de 2008; 42(6): 992-8. [ Links ]
21.Moselakgomo VK, Toriola AL, Shaw BS, Goon DT, Akinyemi O. Índice de massa corpórea, sobrepeso e pressão arterial em escolares na província de limpopo, áfrica do sul. Rev paul pediatr. dezembro de 2012; 30(4): 562-9. [ Links ]
22.Patel ND, Newburn A, Brier ME, Chand DH. Pediatric hypertension: are pediatricians following guidelines? J Clin Hypertens. dezembro de 2016; 18(12): 1230-4. [ Links ]
23.Gerber ZRS, Zielinsky P. Fatores de risco de aterosclerose na infância. Um estudo epidemiológico. Arq Bras Cardiol. outubro de 1997; 69(4): 231-6. [ Links ]
24.Costanzi CB, Halpern R, Rech RR, Bergmann ML de A, Alli LR, Mattos AP de. Associated factors in high blood pressure among schoolchildren in a middle size city, southern Brazil. J Pediatr (Rio J). 7 de agosto de 2009; 85(4): 335-40. [ Links ]
25.Cardoso JL, Leone C. Growth achieved and correlation with blood pressure levels in schoolchildren. Rev Assoc Med Bras. outubro de 2018; 64(10): 896-901. [ Links ]
26.Schommer VA, Barbiero SM, Cesa CC, Oliveira R, Silva AD, Pellanda LC. Excess weight, anthropometric variables and blood pressure in schoolchildren aged 10 to 18 years. Arquivos Brasileiros de Cardiologia [Internet]. 2014 [citado 26 de fevereiro de 2021]; Disponível em: http://www.gnresearch.org/doi/10.5935/abc.20140038 [ Links ]
27.Kumar S, Kelly AS. Review of childhood obesity. Mayo Clinic Proceedings. fevereiro de 2017; 92(2): 251-65. [ Links ]
28.Finn P. Dyslipidemia in overweight and obese school-aged children. NASN School Nurse. setembro de 2015; 30(5): 255-7. [ Links ]
29.Cote AT, Harris KC, Panagiotopoulos C, Sandor GGS, Devlin AM. Childhood obesity and cardiovascular dysfunction. Journal of the American College of Cardiology. outubro de 2013; 62(15): 1309-19. [ Links ]
30.Unger T, Borghi C, Charchar F, Khan NA, Poulter NR, Prabhakaran D, et al. 2020 International Society of Hypertension global hypertension practice guidelines. Journal of Hypertension. junho de 2020; 38(6): 982-1004. [ Links ]
31.Barbalho SM, Oshiiwa M, Sato Fontana LC, Ribeiro Finalli EF, Paiva Filho ME, Machado Spada AP. Metabolic syndrome and atherogenic indices in school children: A worrying panorama in Brazil. Diabetes & Metabolic Syndrome: Clinical Research & Reviews. novembro de 2017; 11: S397-401. [ Links ]
32.Sapunar J, Aguilar-Farías N, Navarro J, Araneda G, Chandía-Poblete D, Manríquez V, et al. Alta prevalencia de dislipidemias y riesgo aterogénico en una población infanto-juvenil. Rev méd Chile. dezembro de 2018; 146(10): 1112-22. [ Links ]
33.Vizentin NP, Cardoso PMS, Maia CAG, Alves IP, Aranha GL, Giannini DT. Dyslipidemia in adolescents seen in a university hospital in the city of rio de janeiro/brazil: prevalence and association. Arquivos Brasileiros de Cardiologia [Internet]. 2018 [citado 26 de fevereiro de 2021]; Disponível em: https://www.scielo.br/scielo.php?pid=S0066-782X2019000200147&script=sci_arttext [ Links ]
34.Gonçalves VS, Duarte EC, Dutra ES, Barufaldi LA, Carvalho KM. Characteristics of the school food environment associated with hypertension and obesity in Brazilian adolescents: a multilevel analysis of the Study of Cardiovascular Risks in Adolescents (Erica). Public Health Nutr. outubro de 2019; 22(14): 2625-34. [ Links ]
35.Pomerantz WJ, Timm NL, Gittelman MA. Injury patterns in obese versus nonobese children presenting to a pediatric emergency department. PEDIATRICS. 1o de abril de 2010; 125(4): 681-5. [ Links ]
36.Chan G, Chen CT. Musculoskeletal effects of obesity. Current Opinion in Pediatrics. fevereiro de 2009; 21(1): 65-70. [ Links ]
37.Yang J, Hu J, Zhu C. Obesity aggravates COVID-19: A systematic review and meta-analysis. J Med Virol. janeiro de 2021; 93(1): 257-61. [ Links ]
38.Hussain A, Mahawar K, Xia Z, Yang W, EL-Hasani S. RETRACTED: Obesity and mortality of COVID-19. Meta-analysis. Obesity Research & Clinical Practice. julho de 2020; 14(4): 295-300. [ Links ]
39.Kelly AS, Fox CK, Rudser KD, Gross AC, Ryder JR. Pediatric obesity pharmacotherapy: current state of the field, review of the literature and clinical trial considerations. Int J Obes. julho de 2016; 40(7): 1043-50. [ Links ]
40.Kelly AS, Barlow SE, Rao G, Inge TH, Hayman LL, Steinberger J, et al. Severe obesity in children and adolescents: identification, associated health risks, and treatment approaches: a scientific statement from the american heart association. Circulation. 8 de outubro de 2013; 128(15): 1689-712. [ Links ]
41.OPAS/OMS Brasil - Obesidade como fator de risco para morbidade e mortalidade: evidências sobre o manejo com medidas não medicamentosas [Internet]. [citado 26 de fevereiro de 2021]. Disponível em: https://www.paho.org/bra/index.php?option=com_docman&view=document&layout=default&alias=1535-obesidade-como-fator-risco-para-morbidade-e-mortalidade-evidencias-sobre-o-manejo-com-medidas-nao-medicamentosas-5&category_slug=serie-uso-racional-medicamentos-284&Itemid=965 [ Links ]
42.Chee Cheong K, Yoon Ling C, Kuang Hock L, Mohd Ghazali S, Chien Huey T, Che Ibrahim M, et al. Association between availability of neighborhood fast food outlets and overweight among 5-18 year-old children in peninsular malaysia: a cross-sectional study. IJERPH. 18 de fevereiro de 2019; 16(4): 593. [ Links ]
43.Fock KM, Khoo J. Diet and exercise in management of obesity and overweight: Diet and exercise for weight management. J Gastroenterol Hepatol. dezembro de 2013; 28: 59-63 [ Links ]
 Correspondence:
Correspondence:
spider@icbusp.org
Manuscript received: November 2020
Manuscript accepted: February 2021
Version of record online: March 2021