Serviços Personalizados
Journal
artigo
Indicadores
Compartilhar
Journal of Human Growth and Development
versão impressa ISSN 0104-1282versão On-line ISSN 2175-3598
J. Hum. Growth Dev. vol.32 no.2 Santo André maio/ago. 2022
https://doi.org/10.36311/jhgd.v32.13317
ORIGINAL ARTICLE
Evaluation of the g473a polymorphism in the lysyl oxidase gene as a risk factor related to the occurrence of breast cancer in Brazilian women
Rodrigo Guilherme Varotti PereiraI; Ricardo Peres do SoutoII; Priscila Larcher LongoIII; César Eduardo FernandesI; Ivo Carelli FilhoI; Rogério Tadeu FeliziI; Melissa Gonzales VeigaI; Emerson de OliveiraI
IDisciplina de Ginecologia, Centro Universitario FMABC - Av. Príncipe de Gales, 821 - Vila Príncipe de Gales, Santo André -SP, Brasil, 09060-650
IIDisciplina de Bioquímica, Centro Universitario FMABC - Av. Príncipe de Gales, 821 - Vila Príncipe de Gales, Santo André - SP, Brasil, 09060-650
IIIUniversidade São Judas Tadeu. - R. Taquari, 546 - Mooca - São Paulo/SP - 03166-000
ABSTRACT
INTRODUCTION: breast cancer is the most diagnosed type of cancer and the leading cause of death among women worldwide. Approximately 1.67 million new cases of breast cancer were diagnosed in 2012, leading to more than half a million deaths. Breast cancer accounted for 11.6% of newly diagnosed cancers (2,089 million) and 9.2% (787,000) of cancer-related deaths for both sexes and at all ages worldwide in 2018
OBJECTIVE: breast cancer, as the most diagnosed carcinoma in the world and the leading cause of death among women, is a morbidity of outstanding importance, and the object of this study is to evaluate the association between the LOX gene G473A (rs1800449) polymorphism and breast cancer occurrence, potentially establishing a new finding in the identification of risks, prevention, and care for a specific group of women
METHODS: in this retrospective cohort study, LOX G473A polymorphism frequency was assessed in 148 women with breast cancer and 245 women without breast cancer. All patients completed a questionnaire to identify possible risk factors and subsequently underwent peripheral blood collection to study the LOX gene. DNA was extracted followed by gene amplification via PCR, and the polymorphism was studied by specific fragment electrophoresis after digestion of the samples with the restriction endonuclease Pstl
RESULTS: this study identified the use of oral contraceptives and family history of breast cancer as risk factors for breast cancer; the G473A polymorphism in LOX was not identified as a risk factor
CONCLUSION: a relationship was not observed between the LOX G473A polymorphism and the occurrence of breast cancer
Keywords: Lysyl Oxidase, Breast Neoplasms, Genetic Polymorphism, Collagen, Elastin.
Authors summary
Why was this study done?
Considering the importance of identifying genetic factors as predisposing factors for the occurrence of different types of cancer, especially being able to offer means of prevention at different levels, the present study was carried out with the objective of identifying the association between the G473A single nucleotide polymorphism of Lysyl Oxidase and the incidence of breast cancer in Brazilian women.
What did the researchers do and find?
In a retrospective study involving 393 Brazilian women, 148 of whom had a confirmed diagnosis of breast cancer and 245 in the control group, the researchers performed DNA extraction, amplification via PCR and the study of the incidence of different polymorphisms, noting that there was no statistically significant relationship between the Lysyl Oxidase gene G473A polymorphism and breast cancer.
What do these findings mean?
The finding means that there is no evidence to link the G473A single nucleotide polymorphism of the Lysyl Oxidase gene to breast cancer.
INTRODUCTION
Breast cancer is the most diagnosed type of cancer and the leading cause of death among women worldwide. Approximately 1.67 million new cases of breast cancer were diagnosed in 2012, leading to more than half a million deaths1. According to data from the International Agency for Research on Cancer of the World Health Organization, breast cancer accounted for 11.6% of new cases of cancers diagnosed (2,089 million) and 9.2% (787,000) of cancer-related deaths for both sexes and all ages worldwide in 20182.
In Brazil, except for non-melanoma skin cancer, breast cancer is the primary type of cancer that affects women, resulting in approximately 25% of new cancer cases (29% prevalence in Brazil) in 2018 and accounting for more than 59,700 new cases, being more frequent in women from South followed by Southeast, Midwest and Northeast regions3. Considering the 2018 data from GLOBOCAN (Global Cancer Data) of the International Agency for Research on Cancer (IARC), the cumulative risk of a woman having breast cancer and her risk of dying from breast cancer in Brazil were 6.8%4 and 1.4%5, respectively.
Considering the significant incidence, prevalence and mortality of breast cancer, knowledge regarding the risk factors for the application of preventive measures is a priority. Among the risk factors for breast cancer are age at menarche, parity, age at first pregnancy, number of living children, absence of breastfeeding history, hormonal contraception, body mass index and obesity, sedentary lifestyle, smoking, exposure to radiation, benign breast disease and changes in breast density, besides genetic factors6.
Regarding risk factors for breast cancer, genetic factors have been widely established variables that play roles in its development7. Among these genetic factors, pathogenic mutations in the BRCA1 and BRCA2 genes confer a high risk for breast cancer, ovarian cancer and contralateral breast cancer; for breast cancer cases, 40% to 87% involve BRCA1 mutations and 18% to 88% involve BRCA2 mutations8,9.
Lysyl oxidase (LOX) catalyzes the oxidative deamination of peptidyl-lysine residues in elastin and lysine precursors and hydroxylysine residues in collagen precursors to form peptidyl-aldehydes, initiating covalent bonds in collagen and elastin crosslinks in the extracellular matrix (ECM)10,11. The covalent bonds in these ECM proteins, facilitated by LOX, are essential for the formation of insoluble collagen and elastic fibers for normal breast development. LOX is a copper-dependent amino acid oxidase enzyme10,11.
One of the main components related to oncogenesis is the remodeling of the ECM, which is defined as a complex structure that provides mechanical support to cells and consists of the interaction of extracellular molecules, including proteoglycans, polysaccharides, fibronectin, laminin and fibers, such as collagen and elastin. During carcinogenesis, ECM composition is modified and stiffens, a process that has been considered a tumor progression factor. This stiffening process has been partially associated with changes in covalent bonds in collagen normally mediated by LOX12.
LOX also has other functions in addition to the maturation of the ECM; it can modify other enzymes and participares in the regulation of cell differentiation, motility, migration and senescence besides gene regulation through transduction and transcription signals10,13. The inhibition of LOX expression alters Ras oncogene activity in fibroblasts, thereby affecting tumor formation. The Ras oncogene is responsible for Ras proteins production, which modify cellular communication in the processes of cell division, differentiation and apoptosis14. In addition, LOX is involved in the recruitment of inflammatory cells to distant sites, which contributes to the formation of the premetastatic niche10,13.
There are approximately 643 SNPs in the LOX gene, and G473A (rs1800449) has the highest polymorphic frequency10,11,15. The LOX G473A SNP is defined as a mutation of the LOX gene that results in the replacement of arginine (Arg) by glutamine (Gln) at residue 158 of the LOX propeptide (LOX-PP); such modification occurs in the presence of the A allele10,11,15.
The presence of the A allele impairs tumor formation suppression performed by LOX-PP and thus has a direct relationship with breast oncogenesis as well as osteosarcoma and ovarian, gastric, colon and rectal carcinomas and increases the risk for coronary disease, keratoconus, retinal detachment, and vitreoretinopathy10,11,15.
In this study, the LOX G473A polymorphism, as an intrinsic factor for carcinogenesis in humans, is analyzed to determine if it can be considered as a risk factor for breast cancer in Brazilian women.
METHODS
In this retrospective cohort study, 393 women were selected between 2013 and 2015 during follow-up at the Mario Covas Hospital outpatient clinic associated with the Mastology Division of the Department of Gynecology and Obstetrics of the Centro Universitário FMABC.
After the research project was approved by the Research Ethics Committee of the FMABC under number 169/2010, the women studied were divided into a control group and a case group. Women with histological confirmation of breast cancer were selected for the case group, totaling 148 participants. A total of 245 women without clinical and radiological signs of breast cancer with consequent clinical propaedeutic and normal mammography were included in the control group.
After a preliminary description of the study and providing clarification when necessary, the included participants signed an informed consent form and responded to a questionnaire to collect the following clinical data: actual age, age at menarche and age at the last menstrual period, number of pregnancies, previous use of hormone medications, breastfeeding, history of smoking and alcohol consumption, and history of endocrine diseases.
Next, venous blood was collected from the women in both groups, and genomic DNA was extracted with Illustra Blood Genomic Prep Mini Spin (GE Healthcare®. After DNA extraction, the LOX G473A polymorphism was detected via PCR using the LoxG473A-Foward 5´-CTCACAGTACCAGCCTCAGCG-3´and LoxG473A-Reverse: 5´-CCAGGTCTGGGCCTTTCATA-3´ (PCR product, 405 bp)16. Amplification of the specific fragment was analyzed by electrophoresis after enzymatic digestion with the restriction endonuclease Pstl.
After restriction digestion, the product was electrophoresed on a 3% NA agarose gel (GE Healthcare®) stained with GelRed® (nucleic acid gel stain, 10,000X, in water) (Biotium). For size determination, 5 μg of a 50 bp DNA ladder molecular weight marker (DNA Express Biotechnology®) was used. The gels were photographed in a MiniBIS Pro Reader (DNR - Bio Imaging System®) under ultraviolet light using GelCapture Version 5.0 (DNR - Bio Imaging System®).
After agarose electrophoresis according to the technique described above, the following standards for analysis were presented: G/G with a single band (405 bp), considered the wild-type standard; A/A with two bands (291 and 114 bp); and G/A with three bands (405, 291, and 114), considered the polymorphic standard.
To verify the association between the study groups and the categorical variables, chi-square test was used, and continuous variables were analyzed using unpaired t-tests.
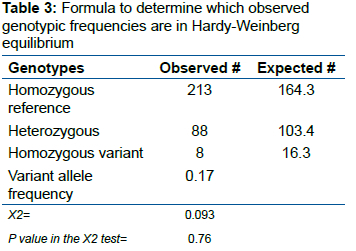
Hardy-Weinberg equilibrium was also tested by the chi-square test and confirmed for the samples.
Once group stratification was confirmed, the relationship between LOX G473A polymorphism and breast cancer was estimated using an odds ratio (OR) obtained by a binary logistic regression model using SPSS version 23.0; the confidence interval adopted was 95% (95 CI). The level of rejection of the null hypothesis was set at 0.05 (α ≤ 0.05).
RESULTS
A total of 393 women participated in the study, of which 148 were in the case group and 245 were in the control group. The clinical and epidemiological characteristics of the participants are provided in table 1. The surgical staging presented by the study participants in the case group are provided in table 2.

For the two groups, a similar profile was found for most of the analyzed variables; however, there were statistically differences significant difference regarding the use of oral hormonal contraceptive and family history of breast cancer.
The genotypes and frequency of alleles in gene equilibrium according to the Hardy-Weinberg ratio (table 3) are provided in table 4. Considering the low incidence of the AA genotype in the study population, we chose to analyze the results by comparing the homozygous reference group (GG) with the polymorphic group (GA + AA).

The presence of the A allele and the GA and AA genotypes in the LOXG473A polymorphism showed no direct association with the occurrence of breast cancer (OR = 0.7735, 95% CI: 0.46 - 1.27, p = 0.314).
DISCUSSION
Breast cancer is the most diagnosed type of cancer in the world and, except for melanoma, the cancer that most affects Brazilian women1-5. The identification of risk factors for breast cancer allows the implementation of population screening and aids in the early diagnosis and actions necessary to minimize the morbidity and mortality of the disease17.
In this regard, the importance of screening through the identification of genetic polymorphisms is highlighted, and the present study is consubstantiated.
Leo et al.18 found increased LOX expression in triple-negative breast cancer (negative for the estrogen, progesterone and Her2 - human epidermal growth factor receptor 2 receptors) and structurally modified ECM. However, in contrast, two studies have shown that a decrease in LOX expression is related to oncogenesis. Csiszar et al.19 observed decreased LOX expression in colon tumors, and Ren et al.20 showed a progressive reduction in LOX expression with the transition from normal to malignant prostatic epithelium.
This apparently contradictory relationship between LOX expression and various types of cancer in which both increased and decreased expression are associated with carcinogenesis can be explained by the embryonic origin of primary tumor cells. Fibrosarcoma, osteosarcoma, rhabdomyosarcoma and renal carcinoma have a mesoderm embryonic origin, while breast carcinoma, melanoma, and choriocarcinoma originate from ectodermal cells and prostate cancer originates form the endoderm. This difference may explain the different LOX expression levels10.
These differences in LOX expression may also be associated with SNPs in which different mutations influence both the action of the active enzyme and its propeptide15,21.
The association between LOXG473A and an increased risk of breast cancer was analyzed in the Chinese population and showed a considerable increase in risk when the A allele was present. The authors evaluated 238 patients with breast cancer and 234 healthy participants. In that study, both GA and AA were associated with an increased risk of breast cancer (OR 2.79, p < 0.001; OR = 2.00, p < 0.001, respectively)11.
Another study with African American women conducted in Boston (USA), with 311 breast cancer patients and 446 participants in the control group, showed no increased risk; however, a possible association was observed regarding the presence of the A allele in patients with estrogen receptor-negative breast cancer compared with patients with estrogen receptor-positive breast cancer, thus suggesting that the risk factors for breast carcinogenesis are multifactorial and dependent on tumor characteristics, such as estrogen receptors22.
When analyzing the profile of the participants in this study, we identified a group of women in the fifth decade of life, with menarche at a mean age of 13 years, with 2 to 3 pregnancies, with the absolute majority being in the postmenopausal period, and with a low incidence of hormone therapy use. This population profile coincides with that observed in the studies described above22,11. Additionally, when evaluating this profile, the use of oral contraceptives and a family history of breast cancer are factors related to the development of breast cancer.
Regarding the comparison between the case and control groups regarding the presence of the A allele, at AA and GA polymorphisms, the present study found no statistically significant difference (OR = 0.7735, 95CI: 0.46 - 1.27, p = 0.314). This lack of a statistical relationship may be explained by the miscegenation of the Brazilian population of this study, which can be similarly observed at USA population and is not observed in a Han Chinese population reported in previous studies22,11.
CONCLUSION
We found no association between the LOX G473A polymorphism and breast cancer in Brazilian women. The use of birth control pills and a family history of breast cancer were associated with breast cancer.
REFERENCES
1.Di Sibio A, Abriata G, Forman D, Sierra MS. Female breast cancer in Central and South America. Cancer Epidemiology 2016; 44: S110-20. https://doi.org/10.1016/j.canep.2016.08.010 [ Links ]
2.Globocan 2018 Latest global cancer data - IARC n.d. https://www.iarc.who.int/infographics/globocan-2018-latest-global-cancer-data/ (accessed April 1, 2022). [ Links ]
3.Institucional. INCA - Instituto Nacional de Câncer 2018. https://www.inca.gov.br/institucional (accessed April 1, 2022). [ Links ]
4.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394-424. https://doi.org/10.3322/caac.21492 [ Links ]
5.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians 2018; 68: 394-424. https://doi.org/10.3322/caac.21492 [ Links ]
6.Rojas K, Stuckey A. Breast Cancer Epidemiology and Risk Factors. Clin Obstet Gynecol 2016; 59: 651-72. https://doi.org/10.1097/GRF.0000000000000239 [ Links ]
7.Goldgar DE, Easton DF, Cannon-Albright LA, Skolnick MH. Systematic population-based assessment of cancer risk in first-degree relatives of cancer probands. J Natl Cancer Inst 1994; 86: 1600-8. https://doi.org/10.1093/jnci/86.21.1600 [ Links ]
8.Mavaddat N, Peock S, Frost D, Ellis S, Platte R, Fineberg E, et al. Cancer risks for BRCA1 and BRCA2 mutation carriers: results from prospective analysis of EMBRACE. J Natl Cancer Inst 2013; 105: 812-22. https://doi.org/10.1093/jnci/djt095 [ Links ]
9.Chen JJ, Silver D, Cantor S, Livingston DM, Scully R. BRCA1, BRCA2, and Rad51 operate in a common DNA damage response pathway. Cancer Res 1999; 59:1752s-6s [ Links ]
10.Friesenhengst A, Pribitzer-Winner T, Schreiber M. Association of the G473A Polymorphism and Expression of Lysyl Oxidase with Breast Cancer Risk and Survival in European Women: A Hospital-Based Case-Control Study. PLoS ONE 2014; 9:e105579. https://doi.org/10.1371/journal.pone.0105579 [ Links ]
11.Ren J, Wu X, He W, Shao J, Cheng B, Huang T. Lysyl oxidase 473 G>A polymorphism and breast cancer susceptibility in Chinese Han population. DNA Cell Biol 2011; 30: 111-6. https://doi.org/10.1089/dna.2010.1098 [ Links ]
12.Jeong YJ, Park SH, Mun SH, Kwak SG, Lee S-J, Oh HK. Association between lysyl oxidase and fibrotic focus in relation with inflammation in breast cancer. Oncology Letters 2018; 15: 2431-40. https://doi.org/10.3892/ol.2017.7617 [ Links ]
13.Payne SL, Fogelgren B, Hess AR, Seftor EA, Wiley EL, Fong SFT, et al. Lysyl oxidase regulates breast cancer cell migration and adhesion through a hydrogen peroxide-mediated mechanism. Cancer Research 2005; 65: 11429-36. https://doi.org/10.1158/0008-5472.CAN-05-1274 [ Links ]
14.Goodsell DS. The molecular perspective: the ras oncogene. Oncologist 1999; 4: 263-4 [ Links ]
15.Wang G, Shen Y, Cheng G, Bo H, Lin J, Zheng M, et al. Lysyl Oxidase Gene G473A Polymorphism and Cigarette Smoking in Association with a High Risk of Lung and Colorectal Cancers in a North Chinese Population. Int J Environ Res Public Health 2016; 13. https://doi.org/10.3390/ijerph13070635 [ Links ]
16.Zhang H-F, Zhao K-J, Xu Y, Hong B, Zhao W-Y, Liu J-M, et al. Lysyl oxidase polymorphisms and ischemic stroke-a case control study. Mol Biol Rep 2012; 39: 9391-7. https://doi.org/10.1007/s11033-012-1803-9 [ Links ]
17.Diretrizes para a detecção precoce do câncer de mama no Brasil. INCA - Instituto Nacional de Câncer 2018. https://www.inca.gov.br/publicacoes/livros/diretrizes-para-deteccao-precoce-do-cancer-de-mama-no-brasil (accessed April 1, 2022). [ Links ]
18.Overexpression of Lox in triple-negative breast cancer. | Sigma-Aldrich n.d. https://www.sigmaaldrich.com/US/en/tech-docs/paper/1413766 (accessed April 1, 2022). [ Links ]
19.Csiszar K, Fong SFT, Ujfalusi A, Krawetz SA, Salvati EP, Mackenzie JW, et al. Somatic mutations of the lysyl oxidase gene on chromosome 5q23.1 in colorectal tumors. Int J Cancer 2002; 97: 636-42. https://doi.org/10.1002/ijc.10035 [ Links ]
20.Ren C, Yang G, Timme TL, Wheeler TM, Thompson TC. Reduced lysyl oxidase messenger RNA levels in experimental and human prostate cancer. Cancer Research 1998; 58: 1285-90. [ Links ]
21.Kirschmann DA, Seftor EA, Fong SFT, Nieva DRC, Sullivan CM, Edwards EM, et al. A molecular role for lysyl oxidase in breast cancer invasion. Cancer Research 2002; 62: 4478-83. [ Links ]
22.Min C, Yu Z, Kirsch KH, Zhao Y, Vora SR, Trackman PC, et al. A loss-of-function polymorphism in the propeptide domain of the LOX gene and breast cancer. Cancer Res 2009; 69: 6685-93. https://doi.org/10.1158/0008-5472.can-08-4818 [ Links ]
 Correspondence:
Correspondence:
Rodrigo Guilherme Varotti Pereira
rodrigo.gineco@hotmail.com
Manuscript received: may 2021
Manuscript accepted: november 2021
Version of record online: june 2022














