Servicios Personalizados
Articulo
Indicadores
Compartir
Psicologia: teoria e prática
versión impresa ISSN 1516-3687
Psicol. teor. prat. vol.19 no.1 São Paulo abr. 2017
http://dx.doi.org/10.5935/1980-6906/psicologia.v19n1p192-207
ARTICLES
HUMAN DEVELOPMENT
Transcranial direct current stimulation in autism: a systematic review
Thiago FernandesI; Ana Luiza Alves DiasII; Natanael Antonio SantosIII
IUniversidade Federal da Paraíba, PB, Brasil
IIUniversidade Federal da Paraíba, PB, Brasil
IIIUniversidade Federal da Paraíba, PB, Brasil
ABSTRACT
Autism spectrum disorder (ASD) is characterized by deficits in social interactions, impairments in language and communication, and highly restrictive behavioral interests. Because of its complex physiopathology, valid and reliable biomarkers are needed for effective diagnosis and treatment, with the goal of symptomatic improvement. Transcranial direct current stimulation (tDCS) is one of the most widely used forms of noninvasive stimulation and may be a promising technique with both diagnostic and therapeutic potential. Using PRISMA methodology, we conducted a review of the contribution of tDCS to ASD treatment. A total of 43 references were found in our literature search, of which six were relevant to this systematic review. Preliminary data suggest an improvement in the behavioral and cognitive symptoms of ASD. However, despite the efficacy of tDCS, some methodological differences were found among the studies, indicating the need for well-designed and controlled studies to confirm the true potential of tDCS for ASD treatment.
Keywords: noninvasive brain stimulation; transcranial direct current stimulation; autism; autism spectrum disorder; systematic review.
Introduction
Autism spectrum disorder (ASD) is a neurodevelopmental disorder that is characterized by impairments in social interactions and communication and restricted patterns of behavior and interests (American Psychiatric Association, 2013). The prevalence of ASD is one in every 110 births (Baron-Cohen et al., 2009; Oberman et al., 2012). Autism spectrum disorder has a higher incidence in children than the combined prevalence of other diseases, such as diabetes, cancer, acquired immunodeficiency syndrome, and Down syndrome (Braun et al., 2014; Oberman et al., 2012).
The diagnosis of ASD is performed clinically through direct observation of the presence of behavioral symptoms that characterize the core of the disorder and through interviews with parents or guardians (American Psychiatric Association, 2013). Some test batteries are used for clinical diagnosis, such as the Childhood Autism Rating Scale (CARS), Autism Treatment Evaluation Checklist (ATEC), Children's Global Assessment Scale (CGAS), and Aberrant Behavior Checklist (ABC; Garfin, McCallon, & Cox, 1988; Geier, Kern, & Geier, 2013).
The CARS is a battery with 15 items that are related to social behavior, emotional responses, the use of objects, body language, adaptation to change, visual responses, perceptual responses, fear, and anxiety (Garfin, McCallon, & Cox, 1988). This scale generally ascertains the severity of autism. The ATEC is a test battery with four subtests: (1) speech/language/communication (14 items, maximum score = 20), (2) social (20 items, maximum score = 40), sensory and cognitive awareness (18 items, maximum score = 36), and (4) physical and behavioral health (25 items, maximum score = 75). A higher score generally indicates lower patient performance. The CGAS assesses the child's psychosocial functioning. Scores range from 1 to 100. Lower scores indicate a more severe disorder (Geier et al., 2013). The ABC consists of 57 items that screen maladaptive behaviors.
There is still no definitive treatment for autism. Most treatments for the core symptoms of ASD are based on behavioral and cognitive interventions (Reichow, 2012). However, these treatments do not produce beneficial effects for severe cases with catatonia, attention-deficit/hyperactivity disorder (ADHD), or high aggressiveness. Pharmacological treatments that use antipsychotics or antidepressants have an adjuvant role but do not effectively reduce the core symptoms of the disorder (Oberman et al., 2012). Many cases present adverse symptoms (e.g., drowsiness, dry mouth, restlessness, insomnia, and greater behavioral impairment; Amatachaya et al., 2015). Therefore, innovative and more effective treatment options are needed.
Neuroimaging studies have investigated the pathophysiology of ASD (Anagnostou & Taylor, 2011; Herbert et al., 2002; Oberman et al., 2012). The findings indicated brain asymmetry that involved a reduction of activity in the left hemisphere. Brain structures in the left hemisphere are related to language, memory, and social functioning. This reduction of activity in the left hemisphere derives from differential synaptic maturation that is caused by microstructural abnormalities, mainly in the left dorsolateral prefrontal cortex (dlPFC) (Chiron et al., 1995; Ozonoff & Miller, 1996; Peterson, Mahajan, Crocetti, Mejia, & Mostofsky, 2015). This brain asymmetry has shed light on the hypothesis that the lateralization of neural function in these circuits can explain the origin of aggressiveness and impairments in social interaction, communication, and language in ASD (Casanova et al., 2013; Said, Egan, Minshew, Behrmann, & Heeger, 2013; Sokhadze et al., 2014).
In addition to behavioral and pharmacological interventions, new procedures that utilize noninvasive brain stimulation techniques, such as transcranial magnetic stimulation (TMS) and transcranial direct-current stimulation (tDCS), can be beneficial for achieving behavioral and cognitive improvements in ASD. These brain stimulation techniques have already been applied for the treatment of other disorders, such as schizophrenia, Alzheimer's disease, and depression, among others (Amatachaya et al., 2015; Bystad et al., 2016; Enticott et al., 2014; Loo et al., 2012). The use of tDCS in children and adolescents with ADHD resulted in behavioral improvements with regard to aggressiveness and the processing of information, such as the detection of environmental stimuli and ability to easily switch between activities (Bandeira et al., 2016; Dambacher et al., 2015).
tDCS has been well studied and is one of the most promising techniques for the treatment of numerous conditions (Kuo, Paulus, & Nitsche, 2014; Tortella et al., 2015). The basis of tDCS is to employ a stimulator that emits continuous low-current electrical currents (0.5-2.0 mA) through two electrodes (cathode and anode), ranging in size from 25 to 35 cm2, that are in contact with the scalp (Stagg & Nitsche, 2011). The flow of the electrical current is able to modulate neuronal excitability, change the resting potential of neurons, and produce effects such as prolonged changes in neuronal excitability, which can be driven by synaptic plasticity. Thus, the stimulation of one area can improve adjacent areas (Stagg & Nitsche, 2011).
Depending on the polarity of the stimulation, the anode can have a depolarizing effect, and the cathode can have an hyperpolarizing effect (Nitsche et al., 2003, 2008). Because of its ability to induce long-term cortical changes, tDCS has been considered a treatment modality for numerous neurological and psychiatric conditions, such as depression (Palm, Hasan, Strube, & Padberg, 2016), schizophrenia (Mondino et al., 2015), and Alzheimer's disease (Bystad et al., 2016).
tDCS can generally have acute or long-lasting effects on cortical function, depending on the parameters that are used for stimulation (e.g., location, frequency, intensity, and repetition of stimulation). The present study critically reviewed evidence of the applicability and efficacy of tDCS for the treatment of ASD.
Methods
Systematic Review
PRISMA guidelines (Liberati et al., 2009; Moher, Liberati, Tetzlaff, & Altman, 2009) were used to guide this systematic review (PROSPERO no. 42017057365). A comprehensive search was conducted in the MEDLINE, Cumulative Index of Nursing and Allied Health (CINAHL), Web of Science, PsycINFO, LILACS, and SciELO databases for articles published until July 2016. The following specific descriptors were used: "transcranial direct current stimulation," "transcranial current stimulation," "micropolarization," "non-invasive brain stimulation," and their abbreviations combined with "autism," "autism spectrum disorder," "Asperger," "Tourette syndrome," and "autistic disorder." The keywords were chosen even in the absence of specific MeSH terms.
Eligibility Criteria
The following eligibility criteria were applied: (1) articles published in English, Portuguese, or Spanish, (2) randomized clinical trials, (3) interventional studies, and (4) books and theses that were fully available. Studies in other languages, electroconvulsive therapy, transcranial magnetic stimulation studies, letters, editorials, studies that assessed conditions other than ASD, and studies that evaluated interventions other than tDCS were excluded.
We extracted the following information from eligible articles: (1) study design, (2) tDCS methodology, (3) side effects, and (4) crucial findings. If insufficient information was available, then the corresponding author was contacted.
Analysis Procedures
During the first screening, two authors (TM or AL) evaluated the titles and abstracts of each citation and excluded irrelevant studies. For each potential study, two authors (NA and TM) examined the full paper and assessed whether the studies met the inclusion criteria. If there was disagreement, a third author (NL) was contacted for consultation until agreement was reached.
Quality Assessment
Articles were evaluated with regard to internal validity (i.e., selection bias, performance bias, attrition bias, and measurement reports) and construct validity (i.e., adequacy of operational criteria used). The quality of the evidence from the studies was assessed using three main measures: (1) limitations (i.e., weaknesses in the study designs), (2) consistency of results, and (3) precision (i.e., generalizability of the findings and sufficient data provided). Studies that had such flaws were excluded.
Results
The initial database search yielded 43 articles. After screening the titles, abstracts, and bibliographies, we considered six articles based on the eligibility criteria for the present systematic review (Figure 1).
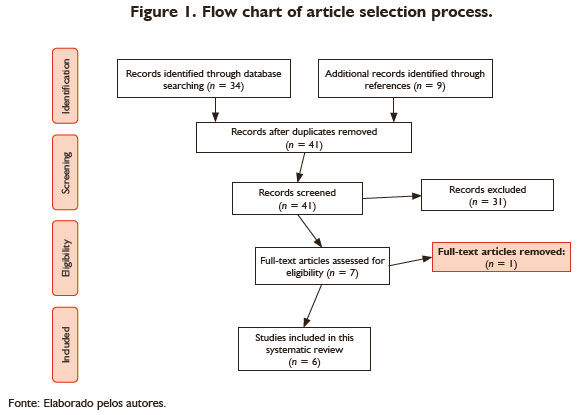
The studies were restricted to randomized clinical trials and case reports. The sample sizes ranged from one to 30 subjects in each study. Table 1 shows the main characteristics of the studies.
The overall analysis was based on 72 patients with ASD, the majority of whom were children. Most of the studies used tDCS together with neuropsychological instruments, such as the ATEC, CARS, CGAS, and ABC, to evaluate differences before and after stimulation. The results indicated that patients with ASD presented improvements in more than half of the studied domains, such as aggressiveness, semantic and lexical domains, and catatonia.
Discussion
Main Findings
The objective of the present systematic review was to evaluate evidence of the effectiveness of tDCS for the treatment of cognitive and behavioral symptoms of ASD. The results of the six studies that were eligible for inclusion in the study reported improvements in ASD symptoms, including improvements in language acquisition, a decrease in hyperactivity, a decrease in aggressiveness, and an increase in activity (in the case of catatonia). The improvement of symptoms and some behaviors occurred even with different stimulation protocols and behavioral indicators, and the effects were relatively long-lasting (Brunoni et al., 2012; Dedoncker, Brunoni, Baeken, & Vanderhasselt, 2016). Some studies reported continual improvements after a follow-up period of 3 and 6 months after stimulation (Costanzo et al., 2015; D'Urso et al., 2014).
A few adverse reactions of stimulation were reported, such as mild irritation of the scalp or in the applied area, but tDCS was well tolerated even after 6 months of consecutive daily applications (Costanzo et al., 2015). The standard setting of stimulation consisted of positioning the electrode over the left dlPFC with the reference electrode outside the scalp in the contralateral deltoid.
This setup is based on neuroimaging findings where the test results that observed irregular current flow between the left dlPFC, medial prefrontal cortex, supplemental motor area, and parietal cortex (Taub, 2015). This irregular flow corroborates the premise that there are dysfunctions in certain cortical regions in ASD patients (Pedapati et al., 2016). Computational models indicated that electrical flow with the commonly used tDCS settings also reaches deep encephalic structures that are involved in the pathophysiology of ASD (Rosenberg, Patterson, & Angelaki, 2015).
Anodic stimulation of the left dlPFC, with the goal of achieving cortical balance in this area because of its extensive connections with other networks that are distributed in the encephalon, was the most used configuration for ASD treatment but not the only one. Thus, the dlPFC appears to be a site of potential interest for studying neuroplasticity in ASD because stimulation can promote a balance between excitation and inhibition, resulting in improvements in neuronal communication (Lee, Lee, & Kim, 2016). Changes in this imbalance may improve cognitive function (Sesarini, 2015), mainly attention and working memory, and the processing of visual information (Vandenbroucke, Scholte, van Engeland, Lamme, & Kemner, 2008). In fact, excitability of the prefrontal cortex improves ASD symptoms, corroborating studies that have reported lower activity in the prefrontal cortex in people with ASD (Gao & Penzes, 2015).
Another line of evidence that supports stimulation of the dlPFC arises from clinical trials that used cathodic rather than anodic stimulation of the left dlPFC, which reversed maladaptive behaviors, such as catatonia and hyperactivity (Costanzo et al., 2015). The therapeutic effects that were observed in the studies that are reviewed herein were related to a reduction of cortical excitability in the dlPFC and the possible intracellular cascade due to inhibitory effects on all neural networks that are interconnected with this region (Gao & Penzes, 2015). The positive effects of stimulation included reductions of dysphoria, irritability, agitation, crying, and behavioral symptoms that were evaluated by the ABC, CARS, CGAS, and ATEC. Neuroimaging studies indicate that the medial prefrontal cortex and medial temporal lobe are also critically involved in the pathophysiology of ASD. Social withdrawal in individuals with ASD is associated with a reduction of dopaminergic signaling in these brain regions, in addition to areas that are interconnected with the dlPFC (Gilbert, Bird, Brindley, Frith, & Burgess, 2008).
Possible Mechanisms of Action of tDCS for ASD Treatment
We provide a summary of the possible mechanisms of action by which tDCS is beneficial for ASD treatment. The specific pathophysiology of this complex disorder, however, remains unknown, and additional mechanisms of action need to be identified. The overall effects of tDCS appear to involve neuronal polarity and neural circuitry (Krause, Márquez-Ruiz, & Cohen Kadosh, 2013; Wokke, Talsma, & Vissers, 2015).
Stimulation Protocols Used
Anodic stimulation increased neuronal excitability, whereas cathodic stimulation decreased neuronal excitability. This shift in polarization likely occurred through the displacement of resting potential, at least when evaluated through the prism of the short-term effects of tDCS (Nitsche & Paulus, 2001). The long-term effects of tDCS have been proposed to result from changes in synaptic function (Medeiros et al., 2012), specifically through changes in the activity of N-methyl-D-aspartate and γ-aminobutyric acid receptors, which can lead to permanent changes in areas that exhibit lower activity. tDCS may also modulate neuroplasticity through brain-derived neurotrophic factor. The hyperactivity of this trophic factor plays a fundamental role in the etiology of autism in early life (Fritsch et al., 2010). The overall impact of tDCS is not restricted to specific sites of application and appears to impact other areas.
Comparisons with Other Studies
We reviewed tDCS and its modes of application for the treatment of ASD. Previous studies have evaluated tDCS for the treatment of ASD based on neuropsychological outcomes. For example, tDCS improved language acquisition (Amatachaya et al., 2015) and operational memory (van Steenburgh, Varvaris, Schretlen, Vannorsdall, & Gordon, 2016). tDCS, together with other forms of micropolarization, appears to improve ASD symptoms.
Relevance of the Study
The relatively few findings that were identified in the present review are nonetheless important for future studies. Indeed, all of the studies discussed herein reported improvements in ASD symptoms after several months of follow-up, demonstrating the durability of the effects of tDCS. Additional controlled and randomized studies with larger sample sizes and different classes of medications should be conducted, and attempts should be made to match the gender of the participants because ASD in females presents more abruptly than in males (Werling & Geschwind, 2013).
At the outset of the present study, we expected to identify only a few studies because the use of tDCS for ASD treatment is still in an incipient stage. Our goal was not to perform a meta-analysis or utilize meta-regression techniques. We instead focused only on highlighting the limitations and contributions of this stimulation technique.
Limitations
Only a few studies have evaluated the effects of tDCS on ASD symptomatology. We employed a robust search strategy. Nonetheless, we might not have identified all studies that are germane to this topic. Despite the importance of case reports, the clinical benefits of tDCS are better understood with the use of randomized controlled trials. We included case reports in the present review because they are oftentimes the first steps toward clinical trials. Although case reports do not provide robust evidence of the efficacy of tDCS for ASD treatment, their findings are important for the design of future studies.
One other limitation is that the studies did not use precisely the same stimulation protocols. Randomized clinical trials using tDCS showed more robust experimental design and replicated the design of studies using TMS, pointing to stimulation in the DLPFC.
Conclusions
The results suggest that tDCS is a promising tool for the treatment of ASD. This conclusion was reached based on diverse studies that used different designs and had different objectives and conditions. This reinforces the use of tDCS as a promising clinical tool for studying and monitoring ASD, especially when considering that it achieves symptomatic improvements when other conventional treatments are ineffective. Protocols that stimulate such regions as the dlPFC can lead to behavioral and cognitive improvements and can serve as research and therapeutic tools.
Unknown are the the specific physiological mechanisms that are altered in ASD and whether these changes can be generalized to the entire population with ASD. Unclear is which region is most affected by ASD and thus which region should be stimulated. Based on neuroimaging studies and the findings presented herein, stimulation of the dlPFC appears to be promising. Further studies need to evaluate the efficacy of tDCS for ASD treatment.
The promising effects of tDCS for ASD indicate which symptoms are best treated using this technique and indicate when to use therapeutic stimulation. This technique needs to be refined so healthcare professionals can determine exactly when stimulation should be applied. Understanding such parameters is crucial from the standpoint of pathophysiology because it may open new avenues for the treatment of refractory or recurrent conditions.
References
Amatachaya, A., Patjanasoontorn, N., Auvichayapat, N., Suphakunpinyo, C., Janjarasjitt, S., Thuleechan, W., ... Auvichayapat, P. (2013). The effects of transcranial direct current stimulation in patients with autism. Srinagarind Medical Journal (SMJ) 28(3),311-319. [ Links ]
Amatachaya, A., Auvichayapat, N., Patjanasoontorn, N., Suphakunpinyo, C., Ngernyam, N., Aree-uea, B., ... Auvichayapat, P. (2014). Effect of anodal transcranial direct current stimulation on autism: a randomized double-blind crossover trial. Behavioural Neurology. Behavioural Neurology, Article ID 173073. doi: 10.1155/2014/173073. [ Links ]
Amatachaya, A., Jensen, M. P., Patjanasoontorn, N., Auvichayapat, N., Suphakunpinyo, C., Janjarasjitt, S., ... Auvichayapat, P. (2015). The short-term effects of transcranial direct current stimulation on electroencephalography in children with autism: a randomized crossover controlled trial. Behavioural Neurology, Article ID 928631. doi: 10.1155/2015/928631. [ Links ]
American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). American Psychiatric Association. Retrieved June 22th, 2017, de http://psychiatryonline.org/doi/book/10.1176/appi.books.9780890425596. [ Links ]
Anagnostou, E., & Taylor, M. J. (2011). Review of neuroimaging in autism spectrum disorders: what have we learned and where we go from here. Molecular Autism, 2(4),1-9. doi: 10.1186/2040-2392-2-4. [ Links ]
Au, J., Katz, B., Buschkuehl, M., Bunarjo, K., Senger, T., Zabel, C., ... Jonides, J. (2016). Enhancing working memory training with transcranial direct current stimulation. Journal of Cognitive Neuroscience, 29(4),1-14. doi: http://doi.org/10.1162/jocn_a_00979. [ Links ]
Baron-Cohen, S., Scott, F. J., Allison, C., Williams, J., Bolton, P., Matthews, F. E., & Brayne, C. (2009). Prevalence of autism-spectrum conditions: UK school-based population study. The British Journal of Psychiatry, 194(6),500-509. doi: http://doi.org/10.1192/bjp.bp.108.059345 [ Links ]
Braun, J. M., Kalkbrenner, A. E., Just, A. C., Yolton, K., Calafat, A. M., Sjödin, A., ... Lanphear, B. P. (2014). Gestational exposure to endocrine-disrupting chemicals and reciprocal social, repetitive, and stereotypic behaviors in 4- and 5-year-old children: the HOME study. Environmental Health Perspectives 122(5),513-520. doi: http://doi.org/10.1289/ehp.1307261 [ Links ]
Breitling, C., Zaehle, T., Dannhauer, M., Bonath, B., Tegelbeckers, J., Flechtner, H.-H., & Krauel, K. (2016). Improving interference control in ADHD patients with trans-cranial direct current stimulation (tDCS). Frontiers in Cellular Neuroscience, 10, Article 72. doi: http://doi.org/10.3389/fncel.2016.00072 [ Links ]
Bystad, M., Grønli, O., Rasmussen, I. D., Gundersen, N., Nordvang, L., Wang-Iversen, H., & Aslaksen, P. M. (2016). Transcranial direct current stimulation as a memory enhancer in patients with Alzheimer's disease: a randomized, placebo-controlled trial. Alzheimer's Research & Therapy, 8(13). doi: http://doi.org/10.1186/s13195016-0180-3 [ Links ]
Casanova, M. F., El-Baz, A. S., Kamat, S. S., Dombroski, B. A., Khalifa, F., Elnakib, A., ... Switala, A. E. (2013). Focal cortical dysplasias in autism spectrum disorders. Acta Neuropathologica Communications, 1,67. doi: http://doi.org/10.1186/2051-5960-1-67 [ Links ]
Costanzo, F., Menghini, D., Casula, L., Amendola, A., Mazzone, L., Valeri, G., & Vicari, S. (2015). Transcranial direct current stimulation treatment in an adolescent with autism and drug-resistant catatonia. Brain Stimulation, 8(6),1233-1235. doi: http://doi.org/10.1016/j.brs.2015.08.009 [ Links ]
D'Urso, G., Bruzzese, D., Ferrucci, R., Priori, A., Pascotto, A., Galderisi, S., ... Bravaccio, C. (2015). Transcranial direct current stimulation for hyperactivity and noncompliance in autistic disorder. The World Journal of Biological Psychiatry: The Official Journal of the World Federation of Societies of Biological Psychiatry, 16(5),361-366. Doi: http://doi.org/10.3109/15622975.2015.1014411. [ Links ]
D'Urso, G., Ferrucci, R., Bruzzese, D., Pascotto, A., Priori, A., Altamura, C. A., ... Bravaccio, C. (2014). Transcranial direct current stimulation for autistic disorder. Biological Psychiatry, 76(5),e5-6. doi: http://doi.org/10.1016/j.biopsych.2013.11.009 [ Links ]
Enticott, P. G., Fitzgibbon, B. M., Kennedy, H. A., Arnold, S. L., Elliot, D., Peachey, A., ... Fitzgerald, P. B. (2014). A double-blind, randomized trial of deep repetitive transcranial magnetic stimulation (rTMS) for autism spectrum disorder. Brain Stimulation, 7(2),206-211. doi: 10.1016/j.brs.2013.10.004. [ Links ]
Fritsch, B., Reis, J., Martinowich, K., Schambra, H. M., Ji, Y., Cohen, L. G., & Lu, B. (2010). Direct current stimulation promotes BDNF-dependent synaptic plasticity: potential implications for motor learning. Neuron, 66(2),198-204. doi: http://doi.org/10.1016/j.neuron.2010.03.035 [ Links ]
Gao, R., & Penzes, P. (2015). Common mechanisms of excitatory and inhibitory imbalance in schizophrenia and autism spectrum disorders. Current Molecular Medicine, 15(2),146-167. [ Links ]
Gilbert, S. J., Bird, G., Brindley, R., Frith, C. D., & Burgess, P. W. (2008). Atypical recruitment of medial prefrontal cortex in autism spectrum disorders: an fMRI study of two executive function tasks. Neuropsychologia, 46(9),2281-2291. doi: http://doi.org/10.1016/j.neuropsychologia.2008.03.025 [ Links ]
Herbert, M. R., Harris, G. J., Adrien, K. T., Ziegler, D. A., Makris, N., Kennedy, D. N., ... Caviness, V. S. (2002). Abnormal asymmetry in language association cortex in autism. Annals of Neurology, 52(5),588-596. doi: http://doi.org/10.1002/ana.10349 [ Links ]
Krause, B., Márquez-Ruiz, J., & Kadosh, R. C. (2013). The effect of transcranial direct current stimulation: a role for cortical excitation/inhibition balance?. Frontiers in Human Neuroscience, 7,602. doi: http://doi.org/10.3389/fnhum.2013.00602 [ Links ]
Kuo, M.-F., Paulus, W., & Nitsche, M. A. (2014). Therapeutic effects of non-invasive brain stimulation with direct currents (tDCS) in neuropsychiatric diseases. NeuroImage, 85 Pt 3,948-960. doi: http://doi.org/10.1016/j.neuroimage.2013.05.117 [ Links ]
Lee, E., Lee, J., & Kim, E. (2016). Excitation/inhibition imbalance in animal models of autism spectrum disorders. Biological Psychiatry, 81(10),838-847. doi: http://doi.org/10.1016/j.biopsych.2016.05.011 [ Links ]
Medeiros, L. F., de Souza, I. C. C., Vidor, L. P., de Souza, A., Deitos, A., Volz, M. S., ... Torres, I. L. S. (2012). Neurobiological effects of transcranial direct current stimulation: a review. Frontiers in Psychiatry, 3,110. doi: http://doi.org/10.3389/fpsyt.2012.00110 [ Links ]
Mondino, M., Brunelin, J., Palm, U., Brunoni, A. R., Poulet, E., & Fecteau, S. (2015). Transcranial direct current stimulation for the treatment of refractory symptoms of schizophrenia. Current evidence and future directions. Current Pharmaceutical Design, 21(23),3373-3383. [ Links ]
Nitsche, M. A., & Paulus, W. (2001). Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology, 57(10),1899-1901. doi: http://doi.org/10.1212/WNL.57.10.1899 [ Links ]
Nitsche, M. A., Liebetanz, D., Lang, N., Antal, A., Tergau, F., & Paulus, W. (2003). Safety criteria for transcranial direct current stimulation (tDCS) in humans. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 114(11),2220-2222. [ Links ]
Nitsche, M. A., Cohen, L. G., Wassermann, E. M., Priori, A., Lang, N., Antal, A., ... Pascual-Leone, A. (2008). Transcranial direct current stimulation: State of the art 2008. Brain Stimulation, 1(3),206-223. doi: http://doi.org/10.1016/j.brs.2008.06.004 [ Links ]
Oberman, L., Eldaief, M., Fecteau, S., Ifert-Miller, F., Tormos, J. M., & Pascual-Leone, A. (2012). Abnormal modulation of corticospinal excitability in adults with Asperger's syndrome. The European Journal of Neuroscience, 36(6),2782-2788. doi: http://doi.org/10.1111/j.1460-9568.2012.08172.x [ Links ]
Oberman, L. M., Rotenberg, A., & Pascual-Leone, A. (2015). Use of transcranial magnetic stimulation in autism spectrum disorders. Journal of Autism and Developmental Disorders, 45(2),524-536. doi: http://doi.org/10.1007/s10803-0131960-2. [ Links ]
Ozonoff, S., & Miller, J. N. (1996). An exploration of right-hemisphere contributions to the pragmatic impairments of autism. Brain and Language, 52(3),411-434. doi: http://doi.org/10.1006/brln.1996.0022 [ Links ]
Palm, U., Hasan, A., Strube, W., & Padberg, F. (2016). tDCS for the treatment of depression: a comprehensive review. European Archives of Psychiatry and Clinical Neuroscience, 266(8),681-694. doi: http://doi.org/10.1007/s00406-016-0674-9 [ Links ]
Pedapati, E. V., Gilbert, D. L., Erickson, C. A., Horn, P. S., Shaffer, R. C., Wink, L. K., ... Wu, S. W. (2016). Abnormal cortical plasticity in youth with autism spectrum disorder: a transcranial magnetic stimulation case-control pilot study. Journal of Child and Adolescent Psychopharmacology, 26(7),625-631. doi: http://doi.org/10.1089/cap.2015.0183 [ Links ]
Peterson, D., Mahajan, R., Crocetti, D., Mejia, A., & Mostofsky, S. (2015). Left-hemispheric microstructural abnormalities in children with high-functioning autism spectrum disorder. Autism Research: Official Journal of the International Society for Autism Research, 8(1),61-72. doi: http://doi.org/10.1002/aur.1413 [ Links ]
Rosenberg, A., Patterson, J. S., & Angelaki, D. E. (2015). A computational perspective on autism. Proceedings of the National Academy of Sciences, 112(30),9158-9165. doi: http://doi.org/10.1073/pnas.1510583112 [ Links ]
Roy, L. B., Sparing, R., Fink, G. R., & Hesse, M. D. (2015). Modulation of attention functions by anodal tDCS on right PPC. Neuropsychologia, 74(jul.),96-107. doi: http://doi.org/10.1016/j.neuropsychologia.2015.02.028. [ Links ]
Rubenstein, J. L. R., & Merzenich, M. M. (2003). Model of autism: Increased ratio of excitation/inhibition in key neural systems. Genes, Brain, and Behavior, 2(5),255-267. [ Links ]
Said, C. P., Egan, R. D., Minshew, N. J., Behrmann, M., & Heeger, D. J. (2013). Normal binocular rivalry in autism: Implications for the excitation/inhibition imbalance hypothesis. Vision Research, 77(jan.),59-66. doi: http://doi.org/10.1016/j.visres.2012.11.002 [ Links ]
Schneider, H. D., & Hopp, J. P. (2011). The use of the Bilingual Aphasia Test for assessment and transcranial direct current stimulation to modulate language acquisition in minimally verbal children with autism. Clinical Linguistics & Phonetics, 25(6-7),640-654. doi: http://doi.org/10.3109/02699206.2011.570852 [ Links ]
Sesarini, C. V. (2015). GABAergic neurotransmission alterations in autism spectrum disorders. Neurotransmitter, 2, Article e1052. doi: http://doi.org/10.14800/nt.1052 [ Links ]
Sokhadze, E. M., El-Baz, A. S., Tasman, A., Sears, L. L., Wang, Y., Lamina, E. V., & Casanova, M. F. (2014). Neuromodulation integrating rTMS and neurofeedback for the treatment of autism spectrum disorder: an exploratory study. Applied Psychophysiology and Biofeedback, 39(3-4),237-257. doi: http://doi.org/10.1007/s10484-014-9264-7 [ Links ]
Stagg, C. J., & Nitsche, M. A. (2011). Physiological basis of transcranial direct current stimulation. The Neuroscientist, 17(1),37-53. doi: http://doi.org/10.1177/1073858410386614 [ Links ]
Steenburgh, J. van, Varvaris, M., Schretlen, D., Vannorsdall, T., & Gordon, B. (2016). Bifrontal transcranial direct current stimulation enhances working memory in adults with high-functioning autism (S9.001). Neurology, 86(16 Supplement), S9.001. [ Links ]
Taub, E. (2015). Neuroplasticity and Neurorehabilitation. Lausanne: Frontiers E-books. [ Links ]
Théoret, H., Halligan, E., Kobayashi, M., Fregni, F., Tager-Flusberg, H., & Pascual-Leone, A. (2005). Impaired motor facilitation during action observation in individuals with autism spectrum disorder. Current Biology, 15(3),R84-R85. doi: http://doi.org/10.1016/j.cub.2005.01.022. [ Links ]
Tortella, G., Casati, R., Aparicio, L. V. M., Mantovani, A., Senço, N., D'Urso, G., ... Brunoni, A. R. (2015). Transcranial direct current stimulation in psychiatric disorders. World Journal of Psychiatry, 5(1),88-102. doi: http://doi.org/10.5498/wjp.v5.i1.88 [ Links ]
Vandenbroucke, M. W. G., Scholte, H. S., van Engeland, H., Lamme, V. A. F., & Kemner, C. (2008). A neural substrate for atypical low-level visual processing in autism spectrum disorder. Brain: A Journal of Neurology, 131(Pt 4), 1013-1024. doi: http://doi.org/10.1093/brain/awm321 [ Links ]
Werling, D. M., & Geschwind, D. H. (2013). Sex differences in autism spectrum disorders. Current Opinion in Neurology, 26(2),146-153. doi: http://doi.org/10.1097/WCO.0b013e32835ee548 [ Links ]
Wokke, M. E., Talsma, L. J., & Vissers, M. E. (2015). Biasing neural network dynamics using non-invasive brain stimulation. Frontiers in Systems Neuroscience, 8, Article 246. doi: http://doi.org/10.3389/fnsys.2014.00246 [ Links ]
 Mailing address:
Mailing address:
Thiago Fernandes
Cidade Universitária, s/n Castelo Branco
João Pessoa PB, 58051-900
Phone: (83) 3216-7337
E-mail: thiagompfernandes@gmail.com
Submission: 22.8.2016
Acceptance: 26.4.2017










 texto en
texto en