Services on Demand
article
Indicators
Share
Revista de Etologia
Print version ISSN 1517-2805
Rev. etol. vol.11 no.1 São Paulo 2012
The reinforcement omission effect on behavioral repertoire of rats with lesions in the basolateral complex and central nucleus of the amygdala
O efeito da omissão de reforço no repertório de ratos com lesão no complexo basolateral e no núcleo central da amígdala
José Lino Oliveira Bueno; Eduardo de Freitas Bernardes; Danielle Marcilio Judice-Daher*
University of São Paulo, Laboratory of Associative Processes, Temporal Control and Memory, Department of Psychology, Faculty of Philosophy, Sciences and Letters of Ribeirão Preto, University of São Paulo, Ribeirão Preto, SP, Brazil
ABSTRACT
Reinforcement omission has been used as a procedure for the evaluation of attentional and motivational processes. Studies show that the activation of some amygdala nuclei may be involved in the modulation of these processes. This study examined the reinforcement omission effects on behavioral repertoire of rats with lesions in the central nucleus and basolateral complex of the amygdala, using classical conditioning and non-contingent reinforcement schemes. Each trial constituted of a 20 second tone, always followed by the delivery of water, in the 19th second. In the sessions involving omission, the water was delivered in half of the trials. The results showed that all groups responded to the omission and only the Basolateral group showed effect in the "Rearing" category, in the period after the omission. These results highlight the need to consider the involvement of a more complex neural network for evaluation of these effects.
Keywords: reinforcement omission, behavioral repertoire, central nucleus of the amygdala, basolateral complex of the amygdala.
RESUMO
A omissão de reforço tem sido usada como procedimento de avaliação dos processos atencionais e motivacionais. Estudos mostram que a ativação de alguns núcleos da amígdala pode estar envolvida na modulação destes processos. O presente trabalho examinou os efeitos da omissão do reforço no repertório comportamental de ratos com lesões no núcleo central e complexo basolateral da amígdala, utilizando-se de esquemas de condicionamento clássico e reforçamento não-contingente. Cada prática constituía de um sinal sonoro de 20 segundos, sempre seguido da liberação de água, no 19º segundo. Nas sessões que envolviam omissão, a água era liberada em metade das práticas. Os resultados mostraram que todos os grupos responderam à omissão e somente o grupo Basolateral apresentou efeito na categoria "Levantar-se", no período após a omissão. Estes resultados apontam a necessidade de se considerar o envolvimento de uma rede neural mais complexa para avaliação destes efeitos.
Palavras-chave: omissão de reforço, repertório comportamental, núcleo central da amígdala, complexo basolateral da amígdala.
Introduction
The reinforcement omission effects (ROEs), which are described through the differences found between the animal's performance immediately after nonreinforcement (N) and after reinforcement (R), has traditionally been interpreted in terms of one among two factors: (1) momentary facilitation after N induced by primary frustration, i.e., an increase in the intensity of the animal's response after the non-reinforced trial due the non-occurrence of an expected event; (2) momentary suppression after R induced by a post-consummatory state, i.e., decrease in the intensity of the animal's response after the reinforced trial due to demotivation caused by the consumption of the reinforcement (Staddon, 1974; Stout, Boughner & Papini, 2003). The reinforcement omission results in a series of effects that are followed by a variety of physiological consequences, and affect the induction, maintenance, facilitation and suppression of a variety of behaviors (Bueno, 1977; Papini & Dudley, 1997), being a indicator of attentional and emotional processes.
Amsel and Roussel (1952), for example, reported that rats run faster on a second runway immediately after reinforcement omission in the goal box of the first runway, than after reinforcement in the same goal box. The authors suggested that there is a behavioral facilitation after N due to a frustration effect, and explained this effect as reï¬ecting motivational reaction of behavior induced by a surprising non-reward. On the other hand, Seward, Pereboom, Butler, and Jones (1957) suggested that the ROEs would not be due to an increase in the speed after N, but a decrease in this speed after R. According to the authors, the food consumption (reinforcement) could induce a transient decrease in motivation to feed.
There are, however, alternative explanations for ROEs that are not only based on motivational consequences. Staddon and Innis (1969), for example, suggested an explanation to the ROEs in terms of reinforcement discriminatory effects. The authors, using fixed-interval (FI) schedules, showed that reinforcement omission produced a reduction in waiting time and a subsequent increase in the response frequencies during the next interval. According to Staddon (1974), the animal learns to not respond when the reinforcement is not available, and thus, by time conditioning learns to not respond in the time period immediately after the delivery of the reinforcement. Consequently, reinforcement acquires a temporal inhibitory control. In this way, the apparent increase in response rates followed by reinforcement omission would result from the removal of the reinforcement inhibitory properties and not due to motivational processes. According to Staddon (1974), the temporal inhibitory control acquired through reinforcement depends on memory and attention properties.
Bueno (1977) studied the ROEs without the procedural limits involving runways or conditions of operant conditioning. The author used a non-contingent reinforcement procedure, registering changes in the rat's behavioral repertoire categories after reinforcement omission. In this experiment, each trial consisted of the presentation of a conditioned stimulus (tone) of 20 seconds, followed by the delivery of an unconditioned stimulus (water) in the 19th second of the tone presentation. When partial reinforcement was introduced, the analysis of the behavioral repertoire during the period after the delivery of reinforcement (post-signal) showed that the rats presented an increase in the rate of responses directed towards the magazine and a decrease in exploratory responses. Right after reinforcement omission, the rats showed levels of exploratory type activity greater than those presented in the post-signal periods after delivery the reinforcement. Therefore, after reinforcement omission, the rats did not show increased responses neither directed to the magazine nor continued to respond as they did before the reinforcement. The author concludes that such effects cannot be indicated by the same generic index of motivational state, since they may be referring to distinct motivational phenomena, which require specific analysis. Therefore, the ROEs must maintain both the motivational/emotional properties and the attentional properties (Judice-Daher, Tavares & Bueno, 2011; Bueno, Judice-Daher & Tavares, 2013).
A possible structure in the nervous system, which could be related to the ROEs would be the amygdala (Figueiredo, 2005). There is evidence supporting the hypothesis that the amygdala is also involved in the modulation of long-term memory storage (McGaugh, Cahill & Roozendaall, 1996). Other studies point to evidence of the role of the amygdala in processing positive emotions, including learning about the beneficial biological value of stimuli (Baxter & Murray, 2002), aspects of associative learning motivated by food and functions often characterized as attentional (Holland & Gallagher, 1999). Each of these functions depends on the individual subsystems of the amygdala, as well as their connections with other brain systems.
Several studies point out the role of the central nucleus of the amygdala (CeA) in attentional processes, using classical conditioning procedures (Holland & Gallagher, 1999; Holland, Han & Gallagher, 2000; Holland, 2006; Holland, 2007). According to Lindgren, Gallagher and Holland (2003), the basolateral complex of the amygdala (BLA) is important for the modification of the motivational significance of events linked to associative learning. Studies have highlighted the role of the BLA and the CeA in the formation and expression of associations between sensory stimuli and reinforcement (Knapska et al., 2008; Tye & Janak, 2007).
Bueno, Judice-Daher and Tavares (2012) examined whether the mechanisms underlying the ROEs would depend on motivational and attentional properties attributing to the activation of the BLA and CeA, respectively. Thus, the ROEs were examined in rats with selective lesions of CeA or BLA and subjected to a fixed-interval schedule (FI 60 min.; Experiment 1) and a fixed-interval with limited-hold signaled schedule (FI 8s LH 2s; Experiment 2). The results from Experiment 1 showed that the group of rats with CeA and BLA lesions, as well as the rats in the sham-operated group, showed increased response rates in intervals following reinforcement omission. On the other hand, the results from Experiment 2 showed that, although the group of rats with CeA and BLA lesions showed increased response rates in periods following reinforcement omission, this increase was smaller than those presented by rats in the sham-operated group. Thus, selective lesions of the amygdala interfered with the ROEs when the partial reinforcement was introduced in the fixed interval with limited-hold signaled schedule, but not in the fixed interval schedule.
More recently, Judice-Daher, Tavares and Bueno (2012) examined whether the underlying mechanisms for ROEs would depend on the different motivational properties attributed to BLA and CeA activation. In this study, lesions of the CeA covered a greater area than in the Bueno et al. study (2012), in which the lesions were made to reach a subregion of CeA related to attention (Holland &Gallagher, 2003). The authors examined whether lesions in the BLA or CeA may interfere with the ROEs employing FI 12s LH 6s signaled schedules and different magnitudes of reinforcements. The results showed that the response rate of rat groups with BLA and CeA lesions was higher after reinforcement omission of smaller magnitude than that observed in rats in their respective sham-operated group. Thus, the lesions of CeA or BLA interfered with the ROEs when smaller magnitude reinforcement was omitted. The results also showed that the rats from all experimental groups, except the rats from the group with lesion of the BLA, showed a higher response rate after the larger reinforcement omission magnitude than after the smaller one.
Bueno, Tavares and Judice-Daher (2012) used a FI 6s LH 6s signaled schedule to examine whether isolated lesions of the BLA or CeA and also larger lesions of the amygdala might affect the ROEs. The results of this study showed that (1) the large neurotoxic lesions of the amygdala (involving both the BLA and the CeA) eliminated the ROEs; (2) bilateral lesions of the BLA seem to make the rats more sensitive to reinforcement omission; the response rates of rat groups with lesion of BLA were higher after the reinforcement omission than those observed in the sham-operated group; and (3) the bilateral lesions of CeA did not interfere with the ROEs. According to Judice-Daher et al. (2012), the larger lesion of the amygdala, involving both the CeA and the BLA, eliminated the ROEs, because it may have affected a larger number of connections between the amygdala and other structures that may also be involved in the modulation of ROEs. These connections may have been preserved when the BLA and CeA were damaged in isolation.
These combined results suggest that nuclei of the amygdala are involved, somehow, in the modulation of ROEs. However, it is necessary to consider the involvement of a more complex neural network for evaluation of ROEs, such as the involvement of connections between the CeA and the BLA with other brain structures (Bueno et al., 2013).
Bueno (1977) showed the relevance of employing analysis of changes in the behavioral repertoire as an adequate strategy for describing the ROEs. However, the lesion effect of different nuclei of the amygdala regarding ROEs employing non-contingent reinforcement and omission of reinforcement within a Pavlovian context has not yet been sufficiently explored. From this perspective, the effects of the lesion of the BLA and CeA on the ROEs, measured from behavioral repertoire observation, could widen understanding of how these areas participate in the modulation of ROEs. Thus, the present study aimed to analyze the ROEs regarding the behavioral repertoire of rats with lesions in the CeA and BLA, within a Pavlovian context, using a procedure of non-contingent reinforcement. With this procedure, it was possible to evaluate how such structures are organized and, also, how these could serve as an clearer indicator of attentional and motivational components that are expressed through changes in the animal's behavioral repertoire. The evaluation of emotional or attentional type responses could serve as an indicator, since they are defined regarding functionality and are not restricted to just one bar-pressing response. This analysis was performed by taking a group of behavioral categories as an object of study, analyzing the relevant characteristics of the behavioral repertoire of the animal that has been subjected to a reinforcement omission procedure. Through this study, it was possible to assess whether lesioned structures can provide indications of attentional and motivational processes in a rat, which can be achieved through the recording of its behavioral repertoire.
Materials and Method
Subjects
36 experimentally naive male Wistar rats, bred in the Central Colony Room of the University of São Paulo in Ribeirão Preto, averaging 450g each and approximately 90 days old at the beginning of the experiment were used. The laboratory colony room in which rats were housed was maintained at an average temperature of 22°C, with the lights kept on daily from 06:30 to 18:30. The experimental sessions took place from 18:30.
Equipment
The behavioral training equipment consisted of 8 experimental boxes (Lafayete model), measuring 20x20x23cm. Each box had lateral walls and a floor (barred) made of stainless steel, front wall, back covered with black cardboard, acrylic roof and a recessed water magazine positioned in the center of the left lateral wall. These boxes were inserted into wooden isolation boxes located on shelves inside a cubicle lined with acoustic Eucatex so as to insulate the boxes from external noise. Ambient lighting (red light) was placed on the roof of the experimental boxes and speakers (20W) producing a tone of 1000 Hz and 30dB were installed within each isolation box. Ambient light (as well as on the bottom of the box) was also covered with black cardboard so that the filming was not impaired. A minicamera was installed on the front side of each box (model VT-786 CCD DN) that filmed the animal's behavior during the session. A video card and a monitoring program were responsible for the recording and storage of data. The experimental control was performed by an interface developed in the Laboratory of Associative Processes, Temporal Control and Memory; and a computer automatically triggered the mechanisms of reinforcement and of stimulus according to a program that had been prepared especially for this experiment. The equipment used for neurosurgery and histology was a stereotaxy apparatus (model David Kopf).
Procedure
The 36 rats were distributed randomly into three groups: Sham-operated (12 animals), lesion of the Central nucleus (12 animals) and lesion of the Basolateral complex (12 animals).
Magazine training and habituation phase
The animals were subjected to daily handling that consisted of removing them from the cage, held on an experimenter's arm for one minute, put on a tray for weighing for approximately 15 seconds and finally introduced to the housing cage. Then the animals were subjected to a regime of water deprivation during which they were kept at 85% of their ad libitum body weight.
During the habituation phase, there was a single 30-minutes session of adjustment during which the mice were able to explore the experimental box. There were also two training sessions at the magazine for a 20 minutes duration or 30 water-delivery trials (whichever occurred first), during which the animals received water every time they had their snout facing the magazine and, in order to receive water again, they had to move away from the magazine.
During the training phase (acquisition and omission), 28 daily sessions were performed, divided into pre and post-lesion training, under two conditions (100% and 50%).
Pre-lesion training
Regarding the trials, each consisted of the presentation of 20 seconds of tone. Approximately 1cc of filtered water was delivered in the 19th second of the sound stimulus presentation. This 100% reinforcement condition (R) was common in all groups at this stage. In total, 20 sessions were performed.
Post-lesion training (retraining)
After the pre-lesion training phase, the animals had an ad libitum diet to reach the ideal weight and thus were subjected to surgery. After the post-surgical recovery period (about 14 days), the animals were again subjected to a deprivation regime to reach the ideal weight. Two sessions of retraining, in the same pre-lesion phase condition, were performed for all groups.
Post-lesion training
In the 50% condition (R and N), following the same conditions as the trials in the 100% group, the water was delivered at the end of only half of the tone presentations. The reinforced (50%-R) and nonreinforced (50%-N) events were randomly distributed during the sessions. This condition was valid for all the groups. Six sessions were held during the post-lesion phase.
Recording of behavioral categories
The behavioral categories were recorded at all phases of the experiment. The category system used for describing the behavioral repertoire is in accordance with the catalog employed in Bueno's experiments (1977). In this study, such categories were grouped as follows:
Category of behaviors directed towards the magazine
Near magazine sitting: the animal, when stationary, remains with its snout in a defined region of 3cm in distance from the magazine.
Magazine sniffing: when stationary, the animal presents whisker and nose movements, accompanied or not by head movements. The animal remains with its snout in a defined region of 3cm in distance from the magazine.
Magazine licking: the animal licks the magazine, when stationary.
Category of exploratory type behaviors
Near magazine sniffing: when the animal keeps both forefeet on the middle of the box floor area next to the magazine; however, this category is not recorded when the animal sniffs its magazine.
Far from magazine sniffing: when the animal keeps both forefeet on the middle of the box floor opposite the magazine.
Rearing: the animal lifts the frontal region of the body, taking the forefeet off the ground, which may or may not be supported on the box walls. These movements are accompanied by head, nose and whisker movements.
Locomotion: with the torso off the floor, the rat performs movements with its four feet (up and down to support itself at another point on the ground) in a coordinated manner that results in body movement across the box floor.
Sitting: The animal keeps four supporting feet on the floor. It may keep its forefeet extended and the ventral torso region off the floor, or its feet and ventral torso region touching the floor.
Grooming: these activities can be of 3 types: (a) the animal licks or nibbles limited areas of the body; (b) the animal lifts a foot from the floor and rubs a surface area of its own body (scratching); or (c) keeping its forefeet off the ground, close to the mouth, licks them, these are then moved to the dorsal region of the head and finally the animal rubs them over the dorsal region towards the ear-nose.
Other: behaviors not covered in the above categories. For example: yawning.
Surgical Procedure
The animals were anesthetized by intraperitoneal injection. The anesthetic was composed of a mixture of 0.8 ml of ketamine hydrochloride (0.028 mg/ml) and 0.7 ml xylazine (3.33 mg/ml). Each mouse received 0.1 ml of anesthetic for each 100 g of body weight. 12 rats received bilateral lesions of the BLA using the following stereotactic coordinates: AP -3.7; ML +- 5.2; DV -8.0 e -8.3. The lesions in the BLA were made using 12.5 mg/ml of N-Methyl-D-aspartic acid (NMDA), infused with a 5 µl Hamilton syringe at a rate of 0.1 ml/min.; 0.2 µl at the deeper site and 0.1 µl at the shallower site. Six sham-operated rats (BLA) received the same surgical treatment, with the exception that the solution was not infused. 12 rats received bilateral lesions of CeA, using the stereotactic coordinates AP-3.2; ML +-4.6; DV-7.7. The lesions of CeA were made using 0.25 ml of 10 mg/ml of ibotenic acid, infused with a 5 µl Hamilton syringe over a period of 2 min, being 0.25 µl per lesioned point. Six sham-operated rats (CeA) received the same surgical treatment, with the exception that no solution was infused. After the surgery, all the rats received a single subcutaneous injection of Banamine anesthetic (2.5 mg/ml; 0.1 ml per 100 g body weight). At the end of the post-lesion stage, the rats were killed by suffocation in carbon dioxide. Then, their encephala were removed from the craniums and went through a post fixing process in formalin (10%). After 24 hours, the brains were transferred to sucrose (30%) where they remained for over 48 hours. The 40 µm coronal histological cuts were made in the cryostat, collected in histological slides and treated using the cresyl violet staining method.
Data analysis
The images of the data referring to the response categories were archived on the computer on which the experiments were executed for later transcription and analysis, through the monitoring system of the program. To ensure the reliability of the collected data, two independent observers recorded the behavioral data in each experiment. At least one observer did not know of the history of the conditioned rats. The data were recorded using the behavioral categories with the observers having to concur with at least 90% of the joint observations. With respect to the recording of behavioral observation, along with their duration, response categories from all the subjects in the different phases were evaluated. The behavioral changes were verified through the differences in the duration of each response category between the groups and periods. The results were subjected to Friedman and Wilcoxon tests to check for possible intragroup differences. For intergroup analysis, the results were subjected to Kruskall-Wallis and Mann-Whitney tests. A significance index of p<0.05 was used for all analyses.
Results
Histological results
One of the 12 rats subjected to surgery from the sham-operated group died. Of the 12 rats sujected to surgery for lesion of the BLA, three died during surgery and four were not included in the analysis of behavioral data due to showing very small BLA lesions. The lesioned rats included in the analysis presented lesions of the anterior and medial portions of the BLA. In no case did any animal subjected to lesion of BLA present lesions of the CeA. Of the 12 rats subjected to surgery for lesion of the CeA, one died and two were not included in the analysis of behavioral data after presenting a unilateral lesion of the CeA. The nine lesioned rats included in the analysis presented lesion to the anterior portion of the CeA and in no case did any of these animals show a lesion of the BLA.
Behavioral results
The data are presented in the figures using average durations of the subject's behavioral categories in order facilitate the reading of the tendencies, but the result description only considers the differences identified as significant by statistical treatment. As there were no statistically significant differences between groups or phases in previous periods, the data presented here only refers to the post-lesion phase. Data from the last six sessions of training indicate the effects of ROEs.
The presented figures are averages of duration (in seconds) of the behavioral categories during the post-signal 1N and post-signal 1R periods.
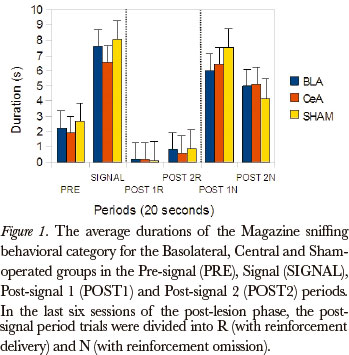
The Friedman test was applied to examine differences between the post-signal 1R, post-signal 2R, post-signal 1N and post-signal 2N periods, in duration rates, for all the groups, in the Magazine sniffing category [Basolateral (P=0.002), Central (P=0.000) and Sham-operated (P=0.000)]. The comparison between pairs (Wilcoxon test), for the differences that occurred between the post-signal 1R and post signal 1N periods, showed significant differences for the Basolateral (P=0.043), Central (P=0.008) and Sham-operated (P=0.008) groups. (Figure 1)
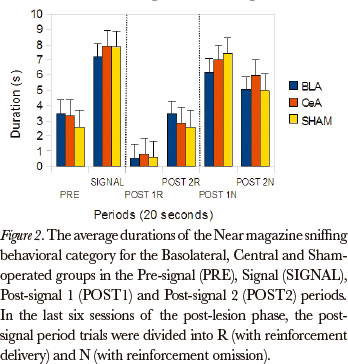
The Friedman test showed significant differences between the post-signal 1R, post-signal 2R, post-signal 1N and post-signal 2N periods, in duration rates, for all groups, in the Near magazine sniffing category [Basolateral (P=0.002), Central (P=0.000) and Sham-operated (P=0.000)]. The comparison between pairs (Wilcoxon test), for the differences that occurred between the post-signal 1R and post-signal 1N periods, showed significant differences for the Basolateral (P=0.043), Central (P=0.008) and Sham-operated (P=0.008) groups. (Figure 2)
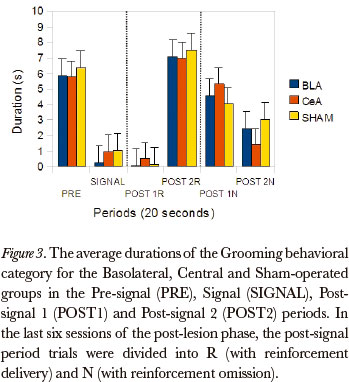
The Friedman test showed significant differences between the post-signal 1R, post-signal 2R, post-signal 1N and post-signal 2N periods, in duration rates, for all groups, in the Grooming category [Basolateral (P=0.005), Central (P=0.000) and Sham-operated (P=0.000)]. The comparison between pairs (Wilcoxon test), for the differences that occurred between the post-signal 1R and post-signal 1N, showed significant differences for the Basolateral (P=0.043), Central (P=0.008) and Sham-operated (P=0.008) groups. (Figure 3)
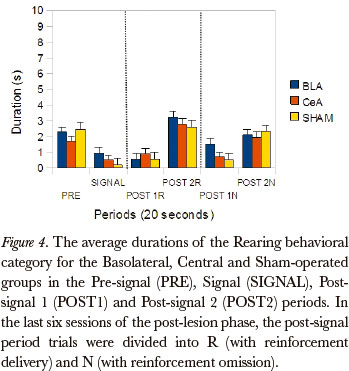
The Friedman test showed significant differences between the post-signal 1R, post-signal 2R, post-signal 1N and post-signal 2N periods, in duration rates, for the Basolateral group in the Rearing category (P=0.003). The comparison between pairs (Wilcoxon test), for the differences that occurred between the post-signal 1R and post-signal 1N periods, showed significant differences (P=0.043). (Figure 4)
The Friedman test showed significant differences between the post-signal 1R, post-signal 2R, post-signal 1N and post-signal 2N periods, in duration rates, for all groups, in the Magazine licking category [Basolateral (P=0.002), Central (P=0.000) and Sham-operated (P=0.000)]. The comparison between pairs (Wilcoxon test), for the differences that occurred between the periods post-signal 1R and post-signal 1N, showed significant differences for the Basolateral (P=0.043), Central (P=0.008) and Sham-operated groups (P=0.008). (Figure 5)
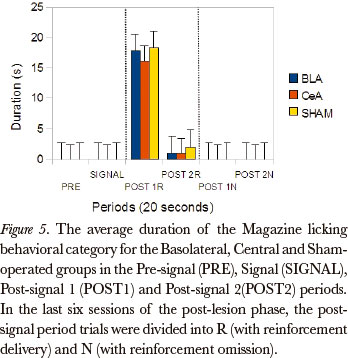
The data from this work, during the phase involving reinforcement omission, also pointed to differences between the groups. The Kruskal-Wallis test showed significant differences in the post-signal 1R period, in duration rates, for Magazine licking (P=0.001). The comparison between the pairs of groups (Mann-Whitney) showed significant differences for this category between the Basolateral and Central (P=0.006) and Central and Sham-operated groups (P=0.002).
The Kruskal-Wallis test showed significant differences in the post-signal 1N period, in duration rates, for Magazine sniffing (P=0.049), Near magazine sniffing (P=0.050) and Rearing (P=0.022). The comparison between the pairs of groups (Mann-Whitney) showed significant differences for the Magazine sniffing category between the Basolateral and Sham-operated groups (P=0.031), Near magazine sniffing between the Basolateral and Sham-operated groups (P=0.039) and Rearing between the Basolateral and Sham-operated groups (P=0.014).
The Kruskal-Wallis test showed significant differences in the post-signal 2N period, in duration rates, for Magazine sniffing (P=0.050) and Grooming (P=0.013). The comparison between the pairs of groups (Mann-Whitney) showed significant differences for the Magazine sniffing category between the Central and Sham-operated groups (P=0.031) and the Grooming category between the Central and Sham-operated groups (P=0.005).
The data, in general, showed that the groups were susceptible to omission, because the behavioral category duration rates during the post-signal N period were higher than the post-signal R period, for all groups: the categories Magazine sniffing, Near magazine sniffing, Magazine licking and Grooming were the most affected by omission.
Discussion
The use of certain response categories serve as an effective tool in the study of classical conditioning. Behavioral studies gain support with the understanding of the animal's behavioral repertoire. The replacement of schedules, in which one or a few responses are recorded by schedules in which the behavioral repertoire is a set of dependent variables, seems to be extremely useful for the study of the underlying processes of behavior (Ades, 1976). Bueno, Figueiredo and Melo (2001) show, for example, that different types of rodents (Wistar rats and Hamsters) have different behavioral strategies when subjected to procedures of conditional discrimination. The results show that the animals have different strategies for the acquisition of a conditional discrimination, as well as highlight the importance of a psychoethological approach for the understanding of complex learning in animals.
With respect to the ROEs, data from this study showed that animals of all experimental groups showed effect of omission. The duration of most behavioral categories were higher after the reinforcement omission, in the post-signal 1N period, than after the delivery of reinforcement, in the post-signal 1R period. For all groups, the categories Magazine sniffing, Near magazine sniffing, Grooming and Magazine licking were affected by the omission. These results support, largely, the data of Bueno (1977) showing, especially, that the duration rates of behavioral categories directed towards the magazine were higher after omission than after the delivery of the reinforcement. Bueno (1977) found an increase in duration of the category Rearing, in the post-N period in relation to the previous signal period, suggesting an omission effect on exploratory activity. In this study, the rats from the Basolateral Group also presented different duration rates for the Rearing category in comparison to the post-signal 1N and post-signal 1R periods. These data suggest that the BLA may be involved in the suppression of exploratory type behaviors. Somehow, the BLA is a behavioral supression component, regardless of its type being exploratory, as in this study, or consummatory (Petrovich & Gallagher, 2003).
The omission can be interpreted as an active agent of behavior modification, as Amsel points out (1958), by affirming that the omission (or, as the author himself says: "frustration effect") is able to create an energizing state of impulse, which can affect the instrumental responses whose consequence was partially suspended, or in other behavioral repertoire responses. There may be, in this case, a correlation with the BLA, since this seems to be related to motivational processes (Holland & Gallagher, 1999; Sah, Faber, Armentia, & Power, 2003). According to this hypothesis, a lesion in this substructure could interfere with the animal's performance, decreasing the ROEs. The energizer aspect, referring to Amsel, was not found in all categories in the present study, but only in those strictly related to the reinforcement source, both for the lesioned animals groups and for the animals in the Sham-operated group. Therefore, the hypothesis of a primary frustration (Stout et al., 2003), i.e. an increase in animal response intensity according to the violation of an expected event, cannot be indiscriminately identified in any behavioral category.
Another explanation of the effects of reinforcement omission ROEs is that they can be understood only as a condition not affected by the effects of consuming the reinforcer. The study by Staddon (1967) showed that when rats are subjected to schedules of reinforcement in FI, the behavioral pattern shown is a post-reinforcement pause followed by increases in response frequency until the next delivery of the reinforcement. The animal may also acquire a temporal inhibitory control, in which case it learns to notrespond in the interval after the delivery of reinforcement, as well as learns to respond when there is an availability of reinforcement (Staddon & Innis, 1969). The fall in performance could occur due to a process of demotivation, as a result of the reinforcement consumption, a post-reinforcement inhibition (post-reinforcement pause), or competition between responses or reponses-criterion (Staddon, 1970). Staddon and Innis (1969) claimed that the mechanisms involved in the omission process have attentional components. In this case, there may be a relationship with the CeA, which seems to be linked to these processes (Holland & Gallagher, 1999; Sah et al., 2003). Thus, a lesion in this substructure could affect the animal's temporal discrimination process, causing the animal to become non-responsive when the reinforcement delivery occurs. The results of this study, however, do not support this hypothesis since the rats from the Central Group, as well as the rats from the Sham-operated group, showed a momentary suppression of some behavioral categories in the acts following reinforcement delivery.
Bueno (1977, 2002) examined the possibility of reinforcement omission being a active modification factor of the behavioral repertoire. In this case, there is a compensatory mechanism that manages the distribution of animal behaviors. When an animal shows changes in frequency of behavior, this change is accompanied by changes in the frequencies of other behaviors: the probability of a determined behavior increase, while the likelihood of incompatible behaviors decreases (Hinde, 1970). Inhibition of one activity may be responsible for the increase in the frequency of another activity. Ades and Bueno (1974) discussed how the plasticity of the behavioral repertoire is an important condition for animal adaptation to variations in the environment: the animal responds to temporal, spatial and predictive relations from the surrounding environment.
The data from this study also showed the ambiguity of the categories, Magazine sniffing and Near magazine sniffing: while Magazine licking, which is a category oriented to the magazine, has a smaller duration in the post-signal N period, Magazine sniffing, which is category oriented towards the magazine, and Near magazine sniffing, which is a type defined as exploratory, present an opposite tendency, of increasing duration. Despite the archives recorded on video showing apparently similar behavioral patterns between the end of the signalization period and the beginning of the omission period (through Magazine sniffing and Near magazine sniffing behavior, which remained over the two periods), these are two distinct and complex processes that require a rapid reorganization of the animal's responses. In studies by Bueno (1977), a sequential analysis was performed to determine which categories occur in sequence, more often than would be expected by chance. The data obtained in this study showed that there is a considerable functional relationship between the Magazine sniffing and Near magazine sniffing categories, during the omission of reinforcement. Magazine sniffing precedes (and interacts strongly with) Near magazine sniffing, at some point of the experiment (post-signal N period).
The omission procedure also depends on the used parameters, because the simple pairing of stimuli does not guarantee that there was conditioning (Henton & Iversen, 1978; Rescorla, 1988), aspects such as duration, intensity and order of presentation of stimuli should be observed and also be considered. The use of behavioral categories (those that occur well before conditioning, when the animal is free in the box to do what it wants) may provide a fundamental taxonomy and a common language for the understanding, prediction and change of behavior, and a way to discover which structures of the brain are possibly responsible for certain behavior. For Olsson, Nevison, Patterson-Kane, Sherwin, Van de Weerd, and Würbel (2003) and Aunger and Curtis (2008), studies involving an ethological approach in the laboratory could help to evaluate (1) the effects of a neutral environment on the behavior and mechanisms that deal with behavior control; (2) the animal's reaction to environmental events that are imposed (3) the functionality and applicability of standardized tests and the potential to develop them considering the specific characteristics of a particular species. Holland (2003) suggested that studies associating neural learning and plasticity should be further developed to strengthen the understanding of the respective areas. Understanding the factors that control omission includes several possible explanations. In animal behavior, the assessment of a class of responses and how a particular response can influence others may offer us a direction of how the behavioral strategies of each species function and how they may have evolved phylogenetically.
A factor to be considered in this study is the inclusion of a surgical procedure, involving lesions of the amygdala substructures that are identified as important in attentional and motivational processes. Behavioral changes resulting from the employment of reinforcement schedules have to take into account, in this study, that these are due, not only to the effects on the lesioned areas, but also as a consequence of surgery. The studied literature does not show an assessment of the behavioral repertoire in the procedure of reinforcement omission in classical conditioning tasks, also involving lesions in substructures of the amygdala.
The attribution of specific nuclei functions of the amygdala was described by Holland e Gallagher (1993), Hatfield, Han, Conley, Gallagher and Holland (1996) and Holland (2006). The authors described the roles assigned to the CeA and BLA, from losses in rat performance with lesions of these nuclei in Pavlovian conditioning procedures. However, these procedures involved not just the omission of an event that was expected, but also the devaluation of reinforcement; being seen as contexts similar to those found in reinforcement omission procedures. In this case, if the ROEs are related to attentional and also motivational aspects, it would be under the control of specific nuclei of the amygdala, since there is support in the literature about the functions of each of these substructures. However, in the Pavlovian procedure used in this study, in which the lesion occurred after the acquiring of the task, in spite of the groups of Basolateral and Central rats having presented the ROEs, some differences were found between lesioned and sham-operated groups, as to the duration rates of some categories, during the post-N and post-R periods. These data suggest that, although the integrity of the BLA and the CeA is not required for animals to exhibit the effects of omission ROEs, these structures could be part of the neural circuitry that modulates the ROEs.
These data accompany the results of studies that have investigated the involvement of amygdala substructures on the ROEs suggesting that the amygdala, in particular the BLA and the CeA, may be part of a more complex brain circuitry involved in the ROEs (Bueno et al., 2013; Judice-Daher et al., 2012).
Acknowlegments
Financial support: This research was funded with a Productivity Scholarship and Research Grant from the National Council for Scientific and Technological Development (CNPq, Brazil) to J. L. O. Bueno; E. F. Bernardes received a Master's Scholarship from the Coordination Improvement of Higher-Education Personnel (CAPES, Brazil) and D. M. Judice-Daher received a Postdoctoral scholarship from the São Paulo Research Foundation (FAPESP).
References
Ades, C. (1976). A observação do comportamento em situações experimentais. Ciência e Cultura, 28, 25-34. [ Links ]
Ades, C. & Bueno J. L. O. (1974). O efeito de um sinal sobre o repertório comportamental do rato. Revista Interamericana de Psicologia, 8, 173-183. [ Links ]
Amsel, A. (1958). The role of frustrative non-reward in non-continuous reward situations. Psychological Bulletin, 55, 102-119. [ Links ]
Amsel, A., & Roussel, J. (1952). Motivational properties of frustration: I. Effect on a running response of the addition of frustration to the motivational complex. Journal of Experimental Psychology, 43(5), 363-368. [ Links ]
Aunger, R., & Curtis, V. (2008). Kinds of behaviour. Biology and Philosophy, 23(3), 317-345. [ Links ]
Baxter, M. G., & Murray, E. A. (2002). The amygdala and reward. Neuroscience, 3, 563-573. [ Links ]
Bueno, J. L. O. (1977). Efeitos da sinalização e do não-reforçamento sobre o repertório comportamental do rato. Ph. D. Thesis, Institute of Psychology, University of São Paulo, São Paulo. [ Links ]
Bueno, J. L. O. (2002). Context effects on temporal control. In: J. A. da Silva, E. H. Mitsushima e N. P. Ribeiro Filho (Orgs.). Fechner Day. (pp. 105-109) Ribeirão Preto: Legis Summa. [ Links ]
Bueno, J. L. O., Figueiredo, T. H., & Melo, F. M. C. (2001). Comparison of pavlovian serial conditional discrimination in rats and hamsters in the same experimental situation. Brazilian Journal of Medical and Biological Research, 34(12), 1595-1602. [ Links ]
Bueno, J. L. O., Judice-Daher, D. M. & Tavares, T. F. (2012). Role of amygdala in the reinforcement omission effects. Psychology & Neuroscience, 5(2), 265-273. [ Links ]
Bueno, J. L. O., Judice-Daher, D. M. & Tavares, T. F. (2013). Neurobiologia dos efeitos de expectativa e omissão de reforço sobre o comportamento. Avances en Psicología Latinoamericana, 31(1), 181-191. [ Links ]
Bueno, J. L. O., Tavares, T. F., & Judice-Daher, D. M. (2012). Neurotoxic amygdala lesions disrupt the reinforcement omission effect. In: 8th FENS Forum of Neuroscience, Barcelona. FENS Forum Abstracts. [ Links ]
Figueiredo, T. H. (2005). Efeito de omissão de reforço em esquemas de intervalo fixo em animais com lesões seletivas do giro denteado hipocampal. Ph. D. Thesis, Institute of Psychology, University of São Paulo, São Paulo. [ Links ]
Hatfield, T., Han, J., Conley, M., Gallagher, M., & Holland, P. C. (1996). Neurotoxic lesions of basolateral, but not central, amygdala interfere with pavlovian second-order conditioning and reinforcer devaluation effects. The Journal of Neuroscience, 16 (16), 5256-5265. [ Links ]
Henton, W. W., & Iversen, I. H. (1978).Classical conditioning and operant conditioning: A response pattern analysis. New York: Springer-Verlag. [ Links ]
Hinde, R. A. (1970) Animal Behavior: a synthesis of ethology and comparative Psychology. New York: McGraw-Hill. [ Links ]
Holland, P. C. (2003). The Psychology and Ethology of Learning. In Irving B. Weiner, (Ed.), Handbook of psychology. (pp. 457-497). Hoboken, NJ: John Wiley & Sons. [ Links ]
Holland, P. C. (2006). Enhanced conditioning produced by surprising increases in reinforcer value are unaffected by lesions of the amygdala central nucleus.Neurobiology of Learning and Memory, 85(1), 30-35. [ Links ]
Holland, P. C. (2007). Disconnection of the amygdala central nucleus and substantia innominata/nucleus basalis magnocellular disrupt performance in a sustained attention task. Behavioral Neuroscience, 121(1), 80-89. [ Links ]
Holland, P. C., & Gallagher, M. (1993). Effects of amygdala central nucleus lesions on blocking and unblocking. Behavioral Neuroscience, 107(2), 235-245. [ Links ]
Holland, P. C., & Gallagher, M. (1999). Amygdala circuitry in attentional and representational processes. Trends in Cognitive Sciences, 3 (2), 65-73. [ Links ]
Holland, P. C., & Gallagher, M. (2003). Double dissociation of the effects of lesions of basolateral and central amygdala on conditioned stimulus-potentiated feeding and Pavlovian-instrumental transfer. European Journal of Neuroscience, 17(8), 1680-1694. [ Links ]
Holland, P. C., Han, J. S., & Gallagher, M. (2000). Lesions of the amygdala central nucleus alter performance on a selective attention task. The Journal of Neuroscience, 20(17), 6701-6706. [ Links ]
Judice-Daher, D. M., Tavares, T. F., & Bueno, J. L. O. (2011). Influence or the reinforcement magnitude on omission effects. Behavioural Processes, 88 (1), 60-62. [ Links ]
Judice-Daher, D. M., Tavares, T. F., & Bueno, J. L. O. (2012). Involvement of the basolateral complex and central nucleus of amygdala in the reinforcement omission effects of different magnitudes. Behavioural Brain Research, 233, 149-156. [ Links ]
Knapska, E., Walasek, G., Nikolaev, E., Neuhäusser-Wespy, F., Lipp, H. P., Kaczmarek, L., et al. (2008). Differential involvement of the central amygdala in appetitive versus aversive learning. Learning and Memory, 13(2), 192-200. [ Links ]
Lindgren, J. L., Gallagher, M., & Holland, P. C. (2003). Lesions of basolateral amygdala impair extinction of CS motivational value, but not of explicit conditioned responses, in Pavlovian appetitive second-order conditioning. European Journal of Neuroscience, 17(1), 160-166. [ Links ]
McGaugh, J. L., Cahill, L., & Roozendaal, B. (1996). Involvement of the amygdala in memory storage: Interaction with other brain systems. Proc. Natl. Acad. Sci., 93(24), 3508-3514. [ Links ]
Olsson, I. A. S., Nevison, C. M., Patterson-Kane, E. G., Sherwin, C. M., Van de Weerd, H. A., & Würbel, H. (2003). Understanding behaviour: the relevance of ethological approaches in laboratory animal science. Applied Animal Behaviour Science, 81(3), 245-264. [ Links ]
Papini, M. R., & Dudley, T. R. (1997). Consequences of surprising reward omissions. Review of General Psychology, 2(1), 175-197. [ Links ]
Petrovich, G. D. & Gallagher, M. (2003). Amygdala subsystems and control of feeding behavior by learned cues. Ann. N.Y. Acad. Sci., 985, 251-262. [ Links ]
Rescorla, R. A. (1988). Pavlovian conditioning: It's not what you think it is. American Psychologist, 43(3), 151-160. [ Links ]
Sah, P., Faber, E. S. L., Armentia, M. L., & Power, J. (2003). The amygdaloid complex: Anatomy and Physiology. Physiological Review, 83(3), 803-834. [ Links ]
Seward, J. P., Pereboom, A. C., Butler, B., & Jones, R. B. (1957). The role of prefeeding in an apparent frustration effect. Journal of Experimental Psychology, 54, 445-450. [ Links ]
Staddon, J. E. R. (1967). Attention and temporal discrimination: factors controlling responding under a cyclic-interval schedule. Journal of the Experimental Analysis of Behavior, 10(4), 349-359. [ Links ]
Staddon, J. E. R. (1970). Temporal effects of reinforcement: A negative "frustration" effect. Learning and Motivation, 1(3), 227-247. [ Links ]
Staddon, J. E. R. (1974). Temporal control, attention and memory. Psychological Review, 5(81), 375-391. [ Links ]
Staddon, J. E. R., & Innis, N. K. (1969). Reinforcement omission on fixed-interval schedules. Journal of the Experimental Analysis of Behavior, 12(5), 689-700. [ Links ]
Staddon, J. E. R., & Simmelhag, V. L. (1971). The "superstition" experiment: a reexamination of its implications for the principles of adaptive behavior. Psychological Review, 78(1), 3-43. [ Links ]
Stout, S. C., Boughner, R. L., & Papini, M. R. (2003). Reexamining the frustration effect in rats: Aftereffects of surprising reinforcement and nonreinforcement. Learning and Motivation, 34(4), 437- 456. [ Links ]
Tye, K. M., & Janak, P. H. (2007). Amygdala Neurons Differentially Encode Motivation and Reinforcement. The Journal of Neuroscience, 27(15), 3937-3945. [ Links ]
Received in November 30, 2012
Accepted in February 10, 2013
* The data of this research were partially presented in the Master's Dissertation by Eduardo de Freitas Bernardes. This research is a continuation of the approach used in the Ph.D. Thesis by J. L. O. Bueno under the advisement of César Ades, which introduces in traditional behavioral studies of rats in experimental boxes the observation of categories of the animal's behavioral repertoire and discusses the data in terms of ethological concepts, in particular the reinforcement-induced behavior. The focus of this research is the study of the reinforcement omission effects, employing this psychoethological strategy..