Serviços Personalizados
artigo
Indicadores
Compartilhar
Psicologia em Pesquisa
versão On-line ISSN 1982-1247
Psicol. pesq. vol.14 no.spe Juiz de Fora 2020
http://dx.doi.org/10.34019/1982-1247.2020.v14.30411
Changes in visual size perception in schizophrenia and depression
Alterações na percepção visual de tamanho na esquizofrenia e depressão
Cambios en la percepción del tamaño en la esquizofrenia y la depressión
Aline Mendes LacerdaI; Maria Lúcia de Bustamante SimasII; Geórgia Mônica Marques de MenezesIII
IUniversidade Federal de Pernambuco - UFPE. E-mail: alinepsicologia@yahoo.com.br. ORCID: https://orcid.org/0000-0001-9861-7241
IIUniversidade Federal de Pernambuco - UFPE. E-mail: maria.simas@ufpe.br ORCID: https://orcid.org/0000-0003-4266-0296
IIIFaculdade do Vale do Ipojuca. E-mail: g_8menezes@hotmail.com ORCID: https://orcid.org/0000-0003-3784-4250
ABSTRACT
The objective of this research was to measure possible changes in visual size perception of patients with depression and schizophrenia. Three groups were compared: Control Group (CG), Schizophrenia Group (SchG) and Depression Group (DepG). The diameter of the first figure seen by the participants in each painting was recorded in degrees of visual angle. The SchG perceived images 1.47 larger than CG and the DepG 1.28 larger than CG, whereas SchG selected images 1.15 larger than DepG, F (2, 57) = 17.677, p < .0001. These findings suggest there are changes in visual size perception related to depression and schizophrenia.
Keywords: Schizophrenia; Depression; Visual perception; Size perception.
RESUMO
O objetivo desta pesquisa foi medir possíveis alterações na percepção visual do tamanho de pacientes com depressão e esquizofrenia. Três grupos foram comparados: Grupo Controle (GC), Grupo Esquizofrenia (SchG) e Grupo Depressão (DepG). O diâmetro da primeira figura vista pelos participantes em cada pintura foi registrado em graus de ângulo visual. O SchG percebeu imagens 1.47 maiores que o GC e o DepG 1.28 maior que o GC, enquanto SchG selecionou imagens 1.15 maiores que DepG, F (2, 57) = 17.677, p < .0001. Esses achados sugerem que há alterações na percepção visual de tamanho relacionadas à depressão e esquizofrenia.
Palavras-chave: Esquizofrenia; Depressão; Percepção visual; Percepção de tamanho.
RESUMEN
El objetivo de esta investigación fue medir posibles cambios en la percepción del tamaño de pacientes con depresión y esquizofrenia. Se compararon tres grupos: Grupo de control (CG), Grupo de esquizofrenia (SchG) y Grupo de depresión (DepG). El diámetro de la primera figura vista por los participantes se registró en grados de ángulo visual. SchG percibió imágenes 1.47 más grandes que CG y DepG 1.28 más grandes que CG, mientras que SchG seleccionó imágenes 1.15 más grandes que DepG, F (2, 57) = 17.677, p < .0001. Hay cambios en la percepción del tamaño relacionados con depresión y esquizofrenia.
Palabras clave: Esquizofrenia; Depresión; Percepción visual; Percepción del tamaño.
The aim of the present research was to measure possible changes in visual size perception of patients with depression and schizophrenia.
Depression and schizophrenia are the main neuropsychiatric disorders in the helm of the world's mental health concerns. Studying their symptoms complements clinical research and contributes to the better understanding of their related cognitive and neurophysiological mechanisms.
A great variety of studies show changes in the visual processing of patients with schizophrenia and depression. Among those are altered perceptual organization (Steven et al., 2020; Panton, Badcock, & Badcock, 2016; Silverstein & Keane, 2011), face perception and recognition (Frassle et al., 2020; Marosi, Fodor, & Csukly, 2019; Stroud et al., 2018; Chen & Ekstrom, 2017; Berg et al., 2016; Maher, Ekstrom, Holt, Ongur, & Chen, 2016; McBain, Norton, & Chen, 2010; Silverstein et al., 2014; Vakhrusheva, et al., 2014), visual integration (Keane, Paterno, Kastner, & Silverstein, 2016; Postmes et al., 2014; Silverstein et al., 2009; Silverstein et al., 2012; Silverstein et al., 2015), visual search (Ferguson & Cane, 2017; Waszczuk, Brown, Eley, & Lester, 2015; Platt, Murphy, & Lau, 2015; Chen, 2011; Dias, Bickel, Epstein, Sehatpour, & Javitt, 2013), spatial frequencies perception (Butler, Thompson, Seitz, Deveau, & Silverstein, 2017; Flevaris, Martínez, & Hilyard, 2014; Graham & Meng, 2011; Green et al., 2009; Kim, Shim, Song, Im, & Lee, 2015; Shoshina, Shelepin, Vershinina, & Novikova, 2015; Silverstein, Demmin, & Bednar, 2017), color perception (Kogata & Iidaka, 2018; Malone et al., 2013; Silver & Bilker, 2015) and movement perception (Jahshan, Wynn, Mathis, & Green, 2015; O´Bryan, Brenner, Hetrick, & O´Donnell, 2014; Golomb et al., 2009) as well as visual illusions (King, Hodgekins, Chouinard, Chouinard, & Sperandio, 2017).
Weckowics and Witney (1960), for instance, studied the effect of visual size perception of the Muller-Lyer illusion in patients with Schizophrenia and found that schizophrenics perceived larger illusion effects than controls, but the group of non-schizophrenic psychiatric patients also showed larger magnitude effects than controls but, in turn, lower than schizophrenics. They could not explain those latter results.
In another study on the Ponzo and Muller Lyer illusions with schizophrenic patients and non-psychiatric "healthy" patients, Shoshina et al. (2011) found opposite results for the Ponzo illusion depending on the number of years of illness. Schizophrenic patients with less than 10 years since diagnosis showed lower sensitivity than healthy patients, whereas the opposite was true for those with more than 10 years diagnostic. These authors also found that those with more than 10 years of illness were more susceptible to the Muller Lyer illusion than those with less than 10 years since diagnosis who, in turn, were more susceptible than healthy patients. But they found gender differences. Apparently, schizophrenic male patients are less susceptible to the Ponzo illusion than females.
We also estimated effects on visual size perception, in analogous way to Shoshina et al. (2011). Instead of illusions, we used paintings by Salvador Dalí. These are rich in images of varying sizes, some superimposed, providing many options of magnitudes for the observers. Our method was initially developed to evaluate patients with schizophrenia, and in the present study we extend this assessment to patients with depression. So, we compared to patients with schizophrenia, depression and healthy volunteers. Based on Simas et al. (2011), our hypothesis was that any effect on size perception would be restricted to schizophrenic patients.
Method
Participants
Sixty volunteers with ages 19-62 yrs old, regardless of gender were divided into three groups: Control Group (CG), Schizophrenia Group (SchG) and Depression Group (DepG).
Volunteers included in DepG were recruited from CAIS Mangabeira and Pan Primavera at João Pessoa-PB, patients included in SchG were recruited from psychiatric clinic from the Hospital das Clínicas from UFPE, at Recife-PE. Volunteers included in CG were recruited from the Tribunal Regional Eleitoral, at João Pessoa-PB. Of those, 20 healthy participants (mean age of 45.11 + 12.70) were randomly assigned to the control group after a screening that excluded from the sample those that marked "c" or "d" in 50% of the questions from the Beck Depression Inventory (BDI).
For the SchG, from 22 patients, 20 were randomly assigned (mean age of 50.10 + 12.7) diagnosed with schizophrenia (F20) according to the International Classification of Diseases, 10th Edition, (ICD-10). Only those whose symptoms were in remission and went to the hospital clinic solely to pick up their prescriptions participated in the study.
The DepG included 20 volunteers (mean age 40.12 + 12.7), diagnosed with depression according to CID-10 as presenting bipolar symptoms without psychosis, currently with depression (F31.4) or with recurrent depression (F33). Volunteers included in this group scored 20 or higher in BDI.
Recruted participants had normal, or corrected to normal, acuity, and volunteers of CG had no use of psychoactive drugs or alcohol 24 hrs prior to test, while patients in both groups were medicated and in remission.
Instruments
The instruments were: (1) individual interview for health condition, familial medical history and sociodemographic data; (2) BDI to assess depression state; (3) Rasquin "E" optotypes; (4) Twenty-four paintings by Salvador Dalí.
Photos of the paintings were printed in 15 x 10 cm glossy paper. These stimuli were selected from a previous study reported by Simas et al. (2011). We also used a partiture stand by Rampazzo & Del Valhe LTDA to place each stimulus, one per trial. Polypropylene material covered the stimulus to allow the pilot markings made by the experimenter over the image selected by each participant.
Procedure
Experiments were run after approval of the Ethics Committee from the "Universidade Federal de Pernambuco, (UFPE)" followed by the signature of the Consent Form by each volunteer. Then, participants were submitted to the BDI and interviewed for assessment of the inclusion/exclusion criteria. The 24 stimuli of Dalí paintings were always placed and presented on the partiture stand in the same order, at 30 cm from the eye. Although there were no time restrictions, volunteers responded within few seconds after each presentation thus the experiment was short and lasted less than 10 minutes on the average. For some in-patients, short intervals were made whenever requested. Volunteers were instructed to indicate the first "at glance" perceived image within each stimulus photograph. The instruction was: "You will see photographs of paintings and, after looking at them, you should point to the first at glance image, or part of an image, you saw". Upon indication of the image, the experimenter would circle the selected one for each of the 24 paintings.
Results
The diameters of the 24 circled images were measured in mm and converted to degrees of visual angle. An ANOVA for repeated measurements with one fator between (Group) and one factor within (Image) showed differences between groups, F(2, 57) = 17.677, p < 0.0001 and between paintings and groups, F(46, 1311) = 4.0091, p < 0.0001.
Table 1 shows mean and sd for each group. The SchG selected images of magnitudes 1.47 fold greater than CG (p < 0.0001) whereas DepG selected those 1.28 fold greater than CG (p < 0.0001). On the other hand, SchG selected images of magnitudes 1.15 fold greater than DepG (p = 0.023). Figure 1 shows mean selected magnitudes for each photograph. It is possible to observe that the CG selected images of smaller magnitudes than DepG that, in turn, selected smaller magnitudes than the SchG.
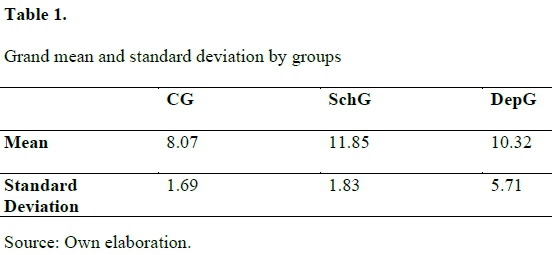
Figure 2 shows frequency of selected magnitudes per group (%) in degrees of visual angle. Thus, 100% from CG, 40% from DepG and 0% from SchG selected magnitudes of 6-9 degrees of visual angle. Forty percent from DepG and 60% from SchG selected magnitudes of 9-12 degrees of visual angle, whereas 20% from DepG and 40% from SchG selected magnitudes greater than 12 degrees of visual angle.
Discussion
These findings suggest that there are indeed changes in the visual size perception in schizophrenia as observed in previous studies (Weckowics & Witney, 1960; Shoshina et al. 2011). However, contrary to our expectation, we did find changes in depression towards the same direction, but to a lesser degree. That is, these appear to be gradual changes of growing magnitudes from the CG, to the DepG to the SchG.
The preference for greater magnitudes found for patients from SchG e DepG implies the existence of some sort of dysfunction(s) in visual reception anf pathways such as contrast sensitivity, tuning to spatial frequency, changes in color and movement perception, etc.
Studies by Kitterle and Selig (1991), Kitterle, Christman and Conesa (1993), Robertson (1995), and Hugdahl and Davidson, (2004) have pointed to the possibility that the two hemispheres differ in temporal and spatial processing of information. Kitterle and colleagues argue that the right hemisphere is biased towards a holistic processing, and this possibly imply an important role of low spatial frequencies, thus greater magnitudes. The left hemisphere, on the other end, tend to process detailed information, consistent with the preferential processing of high spatial frequencies, thus lower magnitudes.
Concerning our findings of higher magnitude preference by patients with schizophrenia and depression, we could suggest that processing by the right hemisphere seems to have some sort of prevalence over the processing by the left hemisphere. This seems not to be the case for the control group.
On the physiological aspect, these findings could be related to dysfunctions in neurotransmitters involved in visual processing, from the retina to associative areas, like dopamine, serotonin and GABA, being dopamine and serotonin the most involved within the retina. Accuracy, noise inhibition and information processing are among the aspects that seem to be related to these neurotransmitters. As for GABA, it is mostly involved at the striate level (Sanacora, Mason, Rothman, & Krystal, 2002; Angelucci & Bullier, 2003) and may contribute to the observed effects.
In sum, this study suggests alteration on visual size perception related to neuropsychiatric disorders. We are conducting further experiments with larger samples improving instructions, instruments and procedure(s).
Acknowledgment
We thank to CAPES and CNPq for support to this research as well as to volunteer patients, healthy controls, and health team from HC-UPFE (Recife-PE), Cais de Mangabeira and Pan Primavera (João Pessoa-PB). We have no conflict of interests.
References
Angelucci, A., & Bullier, J. (2003). Reaching beyond the classical receptive field of V1 neurons: horizontal or feedback axons? Journal of Physiology of Paris, 97, 141-54. https://doi.org/10.1016/j.jphysparis.2003.09.001 [ Links ]
Berg, H. E., Ballard, E. D., Luckenbaugh, D. A., Nugent, A. C., Ionescu, D. F., & Zarate Jr, C. A. (2016). Recognition of emotional facial expressions in anxious and nonanxious depression. Compr Psychiatry, 70, 1-8. https://doi.org/10.1016/j.comppsych.2016.06.007 [ Links ]
Butler, P. D., Thompson, J. L., Seitz, A. R., Deveau, J., & Silverstein, S. M. (2017). Visual Perceptual Remediation for individuals with schizophrenia: Rationale, method, and three case studies. Psychiatr Rehabil J., 40(1), 43-52. https://doi.org/10.1037/prj0000212 [ Links ]
Chen, Y. (2011). Abnormal visual motion processing in schizophrenia: a review of research progress. Schizophrenia Bulletin, 37(4), 709-715. https://doi.org/10.1093/schbul/sbr020 [ Links ]
Chen, Y., & Ekstrom, T. (2017). Perception of faces in schizophrenia: Subjective (self-report) vs. objective (psychophysics) assessments Journal of Psychiatric Research, 76, 136-142. https://doi.org/10.1016/j.jpsychires.2016.02.012 [ Links ]
Dias, E. C., Bickel, S., Epstein, M. L., Sehatpour, P., & Javitt, D. C. (2013). Abnormal task modulation of oscillatory neural activity in schizophrenia. Frontier Psychology, 4, 540. https://doi.org/10.3389/fpsyg.2013.00540 [ Links ]
Ferguson, H. J., & Cane, J. (2017). Tracking the impact of depression in a perspective-taking task. Scientific reports, 7, 14821. https://doi.org/10.1038/s41598-017-13922-y [ Links ]
Flevaris, A., Martínez, A., & Hilyard, S. A. (2014). Attending to global versus local stimulus features modulates neural processing of low versus high spatial frequencies: an analysis with event-related brain potentials. Frontier Psychology, 5, 277. https://doi.org/10.3389/fpsyg.2014.00277 [ Links ]
Frassle, S., Marquand, A. F., Schmaal, L., Dinga, R., Veltman, D. J., Wee, N. J. A. V., ... Stephan, K. E. (2020). Predicting individual clinical trajectories of depression with generative Embedding. Neuroimage Clin., 26, 102213. https://doi.org/10.1016/j.nicl.2020.102213 [ Links ]
Golomb, J. D., McDavitt, J. R., Ruf, B. M., Chen, J. I., Saricicek, A., Maloney, K. H., ... Bhagwagar Z. (2009). Enhanced visual motion perception in major depressive disorder. The Journal of Neuroscience, 29(28), 9072-9077. https://doi.org/10.1523/JNEUROSCI.1003-09.2009 [ Links ]
Graham, D., & Meng, M. (2011). Altered spatial frequency contents in paintings by artists with schizophrenia. Iperception, 2(1), 1-9. https://doi.org/10.1068/i0391 [ Links ]
Green, M. F., Butler, P. D., Chen, Y., Geyer, M. A., Silverstein, S., Wynn, J. K., ... Zemon, V. (2009). Perception Measurement in clinical trials of Schizophrenia: Promising paradigms from CNTRICS. Schizophrenia Bulletin, 35(1),163-181. https://doi.org/10.1093/schbul/sbn156 [ Links ]
Hugdahl, K., & Davidson, R. J. (2004). The assimmetrical brain. Cambridge, UK: A Bradford book. [ Links ]
Jahshan, C., Wynn, J. K., Mathis, K. I., & Green, M. F. (2015). The neurophysiology of biological motion perception in schizophrenia. Brain and Behaviour, 5(1), 5-84. https://doi.org/10.1002/brb3.303 [ Links ]
Keane, B. P., Paterno, D., Kastner, S., & Silverstein, S. M. (2016). Visual integration dysfunction in schizophrenia arises by the first psychotic episode and worsens with illness duration. J Abnorm Psychol., 125(4), 543-549. https://doi.org/10.1037/abn0000157 [ Links ]
Kim, D. W., Shim, M., Song, M. J., Im, C. H. & Lee, S. H. (2015). Early visual processing deficits in patients with Schizoprenia during spatial frenquency-dependent facial affect processing. Schizophrenia Research, 161(2-3), 314-321. https://doi.org/10.1016/j.schres.2014.12.020 [ Links ]
King, D. J., Hodgekins, J., Chouinard, P. A., Chouinard, V. A., & Sperandio, I. (2017). A review of abnormalities in the perception of visual illusions in schizophrenia. Psychonomic Bulletin & Review, 24(3), 734-751. https://doi.org/10.3758/s13423-016-1168-5 [ Links ]
Kitterle, F. L., Christman, S., & Conesa, J. S. (1993). Hemispheric differences in the interference among components of compound gratings. Perception & Psychophysics, 54, 785-793. [ Links ]
Kitterle, F. L., & Selig, L. M. (1991). Visual field effects in the discrimination of sine-wave gratings. Perception & Psychophysics, 50, 15-18. [ Links ]
Kogata, T., & Iidaka, T. (2018). A review of impaired visual processing and the daily visual world in patients with schizophrenia. Nagoya J Med Sci. 80(3): 317-328. https://doi.org/10.18999/nagjms.80.3.317 [ Links ]
Maher, S., Ekstrom, T., Holt, D., Ongur, D., & Chen, Y. (2016). The core brain region for face processing in schizophrenia lacks face selectivity. Schizophrenia Bulletin, 42(3), 666-674. https://doi.org/10.1093/schbul/sbv140 [ Links ]
Malone, J. C., Stein, M. B., Slavin-Mulford, J., Bello, I., Sinclair, S. J., & Blais, M. A. (2013). Seeing red: affect modulation and chromatic color responses on the Rorschach. Bulletin of the Menninger Clinic, 77(1), 70-93. https://doi.org/10.1521/bumc.2013.77.1.70 [ Links ]
Marosi, C., Fodor, Z., & Csukly, G. (2019). From basic perception déficits to facial affect recognition impairments in schizophrenia. Scientific Reports, 9, 8958. https://doi.org/10.1038/s41598-019-45231-x [ Links ]
McBain, R., Norton, D., & Chen, Y. (2010). Differential roles of low and high spatial frequency content in abnormal facial emotion perception in Schizophrenia. Schizophrenia Research, 122(1-3), 142-149. https://doi.org/10.1016/j.schres.2010.03.034 [ Links ]
O'Bryan R. A., Brenner, C. A., Hetrick, W. P., & O'Donnell, B. F. (2014). Disturbances of visual motion perception in bipolar disorder. Bipolar Disorders, 16(4), 354-365. https://doi.org/10.1111/bdi.12173. [ Links ]
Panton, K. R.; Badcock, D. R.; Badcock, J. C. (2016). A metaanalysis of perceptual organization in schizophrenia, schizotypy, and other high-risk groups based on variants of the embedded figures task. Frontiers in Psychology, 7(237). https://doi.org/10.3389/fpsyg.2016.00237 [ Links ]
Platt, B.; Murphy, S. E.; Lau, J. Y. F. (2015). The association between negative attention biases and symptoms of depression in a community sample of adolescents. PeerJ. https://doi.org/10.7717/peerj.1372
Postmes, L., Sno, H. N., Goedhart, S., Van der Stel, J., Heering, H. D., & de Haan, L. (2014). Schizophrenia as a self-disorder due to perceptual incoherence. Schizophrenia Research, 152(1), 41-50. https://doi.org/10.1016/j.schres.2013.07.027 [ Links ]
Robertson, L. C. (1995). Hemispheric specialization and cooperation in processing complex visual patterns. In F. L. Kitterle (Ed.). Hemisfheric communication: Mechanism and models. New Jersey: Lawrance Erlbaum Associates. [ Links ]
Sanacora, G., Mason G. F., Rothman D. L., & Krystal J. H. (2002). Increased occipital cortex GABA concentrations in depressed patients after therapy with selective serotonin reuptake inhibitors. The American Journal of Psychiatry, 159, 663-665. https://doi.org/https://doi.org/10.1176/appi.ajp.159.4.663 [ Links ]
Silverstein, S. M., Berten, S., Essex, B., Kovács, I., Susmaras, T., & Little, D. M. (2009). An fMRI examination of visual integration in schizophrenia. Journal of Integrative Neuroscience, 8(2), 175-202. https://doi.org/10.1142/S0219635209002113 [ Links ]
Silverstein, S. M., Demmin, D. L. & Bednar, J. A. (2017). Computational modeling of contrast sensitivity and orientation tuning in first-episode and chronic Schizophrenia. Computational Psychiatry, 1, 102-131. https://doi.org/10.1162/CPSY_a_00005 [ Links ]
Silverstein, S. M., Harms, M. P., Carter, C. S., Gold, J. M., Keane, B. P., MacDonald, A., … Barch, D. M. (2015). Cortical contributions to impaired contour integration in Schizophrenia. Neuropsychologia, 75, 469-480. https://doi.org/10.1016/j.neuropsychologia.2015.07.003 [ Links ]
Silverstein, S. M., & Keane, B. P. (2011). Perceptual organization impairment in schizophrenia and associated brain mechanisms: review of research from 2005 to 2010. Schizophrenia Bulletin, 37(4), 690-699. https://doi.org/10.1093/schbul/sbr052 [ Links ]
Silverstein, S. M., Keane, B. P., Barch, D. M., Carter, C. S., Gold, J. M., Kovacs, I., … Strauss, M. E. (2012). Optimization and validation of a visual integration test for schizophrenia research. Schizophrenia Bulletin, 38(1), 125-134. https://doi.org/10.1093/schbul/sbr141 [ Links ]
Silverstein, S. M., Keane, B. P., Papathomas, T. V., Lathrop, K. L, Kourtev, H., Feigenson, K., … Paterno, D. (2014). Processing of Spatial-frequency altered faces in Schizophrenia: effects of illness phase and duration. Plos One, 9(12), e114642. https://doi.org/10.1371/journal.pone.0114642 [ Links ]
Simas, M. L. B., Nogueira, R. M. T. B. L., Menezes, G. M. M., Amaral, V. F., Lacerda, A. M., & Santos, N. A. (2011). O uso de pinturas de Dalí como ferramenta para avaliação das alterações na percepção de forma e tamanho em pacientes esquizofrênicos. Psicologia USP, 22(1), 66-80. https://doi.org/10.1590/S0103-65642011005000006 [ Links ]
Steven, M., Silverstein, A. R., Seitz, A. O., Ahmed, A. O., Thompson, J. L., Zemon, V., ... Butler, P. D. (2020). Development and evaluation of a visual remediation intervention for people with schizophrenia. J Psychiatr Brain Sci., 5. https://doi.org/10.20900/jpbs.20200017 [ Links ]
Shoshina, I. I., Perevonzchikova, I. N., Konkina, S. A., Pronin, S. V., Shelepin, I. E., & Bendera, A. P. (2011). Features of perception of lenght of segments under conditions of ponzo na Myller-Lyer in schizophrenia. Zh Vyssh Nerv Deiat Im I P Pavlova, 61(6), 697-705. [ Links ]
Shoshina, I. I., Shelepin, Y. E., Vershinina, E. A., & Novikova, K. O. (2015). The spatial-frequency characteristics of the visual system in schizophrenia. Human Physiology, 41, 251-260. [ Links ]
Silver, H., & Bilker, W. B. (2015). Colour influences perception of facial emotions but this effect is impaired in healthy ageing and schizophrenia. Cognitive Neuropsychiatry, 20(5), 438-455. https://doi.org/10.1080/13546805.2015.1080157 [ Links ]
Stroud, J. B., Freeman, T. P., Leech, R., Hindocha, C., Lawn, W., Nutt, D. J., ... Harris, R. L. C. (2018). Psilocybin with psychological support improves emotional face recognition in treatment-resistant depression. Psychopharmacology, 235, 459-466 https://doi.org/10.1007/s00213-017-4754-y [ Links ]
Vakhrusheva, J., Zemon, V., Bar, M., Weiskopf, N. G., Tremeau, F., Petkova, E.... Butler, P. D. (2014). Forming first impressions of other in Schizophrenia: Impairment in fast processing and in use of spatial frequency information. Schizophrenia Research, 160(1-3), 142-149. https://doi.org/10.1016/j.schres.2014.10.012 [ Links ]
Waszczuk, M. A., Brown, H. B., Eley, T. C., & Lester. K. J. (2015). Attentional control theory in childhood: enhanced attentional capture by non-emotional and emotional distractors in anxiety and depression. Plos One, 10(11), 1-14. https://doi.org/10.1371/journal.pone.0141535 [ Links ]
Weckowics, T. E., & Witney, G. (1960). The Muller-Lyer in schizophrenia patients. The Journal of Mental Science, 106, 1002-1007. https://doi.org/10.1192/bjp.106.444.1002 [ Links ]
Recebido em: 30/04/2020
Aceito em: 02/09/2020














