INTRODUCTION
The acute respiratory tract infection epidemic, known as COVID-19, started in December 2019 and its most frequent clinical symptoms are respiratory, such as cough, fever and difficulty breathing. Nevertheless, the clinical aspects of the new corona virus infection (SARS-CoV-2) are wild heterogeneous, ranging from a cold to serious pneumonia1. The elderly and individuals with comorbidities (hypertension, chronic obstructive pulmonary disease, diabetes and cardiovascular disease) rapidly evolved to acute respiratory syndrome, septic shock, severe metabolic acidosis and coagulation disfunction, leading to worsening of the condition and death2,3.
Because of the rapid and global spread, the World Health Organization (WHO) declared in March 11th 2020 the new coronavirus disease (2019-nCoV), a pandemic4. However, the divulged data on the number of cases may not represent the actual reality; there are doubts about the COVID-19 mortality rate, because these rates vary between countries. One reason for this variation is the lack of resources such as tests to confirm the infection5. Furthermore, there is an alert for asymptomatic cases and patients with mild symptoms, who may not be tested and consequently will not be identified and included in the statistic of COVID-19 confirmed cases. This situation brings to light the necessity of further knowledge on SARS-CoV-2 physiopathology, such as blood profile and possible predictors6,7.
Among the studied predictors are the vital hematological parameters which include leukocyte, neutrophil, lymphocyte and platelet counts and the proportion between the neutrophil and lymphocyte ratio. Some hematological parameters, like the ones mentioned above, can help in predicting and monitoring the progression of several diseases8,9.
Just as occurred in severe acute respiratory syndrome (SARS) in 2003 in China, and in Middle East respiratory syndrome (MERS) in 2012, there are alterations in circulating blood cells, besides abnormalities in function and in lymphocyte counts. Leucopenia, neutrophilia and thrombocytopenia were blood alterations frequently detected in positive patients2,10-12.
The Lymphopenia may be related to an immune response deficiency toward the virus. Neutrophilia can indicate increased cytokines in a hyper-inflammatory state with of abnormalities in cytoplasmatic and nuclear morphology, Ranging from hypo-segmented nucleus to apoptosis11. This increases neutrophil/lymphocyte ratio (NLR) which is associated with higher inflammatory cytokines (IL-2, IL-6 and IL-10) and higher IgG values, leading to serious cases and worse prognosis13. Regarding platelet count which is drastically decreased in severe patients and especially in non-survivors, a condition thought to result from platelet activation, aggregation, and adhesion as well as increased consumption of these plasmatic elements14.
Brandon Michael Henry and collaborators observed that severe cases presented a slight increase in leukocyte count while fatal patients showed a significantly higher increase of this parameter. Decreases in hemoglobin and eosinophil counts are also reported15. Another study observed that the increase in the Red Cell Distribution Width (RDW) is associated with higher mortality in hospitalized patients with COVID-19, no matter the age. Nevertheless, higher RDW levels at the moment of hospital admission predicted higher mortality rate16. Moreover, in severe cases, hemophagocytic syndrome was also observed to be caused by macrophage activity17. Lee et al. (2021) conducted a meta-analysis, demonstrating the potential usefulness of RDW measurement in predicting COVID-19 mortality and disease severity, highlighting its significance as a prognostic biomarker18. Similarly, they retrospectively studied the clinical outcomes of hospitalized COVID-19 patients based on their RDW values, revealing a strong association between elevated RDW and unfavorable outcomes in this patient population19.
It is known that hematological manifestations are common in COVID-19 disease, but there are still few data available about the prevalence and clinical significance of anemia and other alterations in this disease. In most cases, anemia was mild and was caused by an inflammatory process, associated or not with iron and vitamins deficiency. Therefore, this condition can have a negative impact on patients’ quality of life, so monitoring the Mean Corpuscular Volume (MCV), Mean Corpuscular Hemoglobin (MCM) and Mean Corpuscular Hemoglobin Concentration (MCHC) is of great importance. Even so, data is still inconsistent, and more observations need to be made20.
Despite of the differing studies over hematological abnormalities, we believe there is still space to explore these parameters given their importance in the current pandemic scenario and the necessity of further knowledge of its repercussion. Furthermore, it is important to consider and study other parameters such as RDW or platelet to lymphocyte ratio (PLR), which is already used for acute pancreatitis, breast cancer and acute myocardial infarction prognostic and as a severity predictor for cirrhosis associated with chronic hepatitis and fibrosis21.
Considering that a hemogram is a low cost, minimally invasive exam, which is already used as a prognostic tool for acute pancreatitis, breast cancer and acute myocardial infarction. The objective of the present study was mapping the major hematological alterations observed in hospitalized COVID-19 patients, regardless of the severity of the disease, and to identify the most prevalent hematimetric changes in these patients. The results of this study will be fundamental to strengthen the diagnosis in SUS and assist in the monitoring of COVID-19 inpatients, besides aiding medical decisions. This evaluation can also cooperate with possible drug or supplementation studies to improve immunological response and patient recovery.
METHODS
Delineation
This is a cross-sectional study which evaluates hematological variables in 200 patients diagnosed with COVID-19, in comparison with 200 negative patients, between July 2020 and January 2021, before the vaccination period started. This study was conducted during the first wave of COVID-19 in the city of São Bernardo do Campo and Santo André. All participants received care in field hospitals; therefore, patient inclusion followed a nonprobability or nonrandom sampling, considering the pandemic state. As a result, there was no distinction based on the severity level or mortality of the included patients22. Reports were collected through the Matrix FMABC platform, in which it is possible to obtain data on all exams performed in Laboratório de Análises Clínicas of Centro Universitário FMABC, taken from samples acquired from health centers in the municipalities of São Bernardo do Campo and Santo André.
Eligibility Criteria
We analyzed hemogram parameters up to 9 days after a positive result for COVID-19, as the disease typically spans a 14-day period, and symptoms typically manifest around the 5th day of infection, prompting individuals to seek healthcare services for testing23. Therefore, within the first 9 days after a positive test, patients are still in the active phase of the disease. This study did not differentiate severity or mortality data. Given the convenience sample nature, we did not perform a matching test between female and male participants. Age and sex were not distinguished among the participating groups, as they were selected based on demand in the health services of Santo André and São Bernardo do Campo.
COVID-19 Positive Group: Patients suspected of coronavirus infection confirmed by a positive RT-qPCR test, with no age or sex distinctions. COVID-19 Negative Group: Patients suspected of coronavirus infection ruled out by a negative RT-qPCR test, with no age or sex distinctions. Exclusion criteria: patients with previous coronavirus infection, patients who did not fit the inclusion criteria, patients with hemograms performed over 9 days from the COVID-19 detection RT-qPCR test.
Hematological evaluation
Erythrogram, leukogram and platelet counts were performed by flow cytometry in a ABX Pentra DF 120™ device, following good practices in clinical analysis. The analyzed parameters were specific: erythrocytes (Reference value (RV): for men 4.5 - 5.5 million/mm3 and for women 4-5 million/mm3), hemoglobin (RV: for men 13-17 g/dL and for women 12-15 g/dL dL), VCM (RV: 80-100 μm3), MCH (RV: 27-32 pg), MCHC (RV: 31.5-36 g/dL), hematocrit (RV: 36 -46%), RDW (RV: 12-15%), platelets (RV: 150-400 103 mm3), MPV (RV: 6-10 μm3), leukocytes (RV: 4, 5-11 103 mm3), neutrophils (RV: 2-8 103 mm3), lymphocytes (RV: 1-5 103 mm3) and the ratio between neutrophils and lymphocytes, which is a marker for reflecting the inflammatory status of the patient and serves as a prognostic factor24,25.
Ethical aspects
This study is in accordance with Process N° 466 de 12/12/2012, from Conselho Nacional de Saúde – CNS, which regulates research with humans. The project was approved by Comitê de Ética em Pesquisa - CEP from FMABC, nº 4.427.013, as laid down in the 1964 Declaration of Helsinki.
Statistical analysis
Data were expressed as mean ± standard deviation (SD). The unpaired Student t-test was performed on normal distribution values and the Mann Whitney test was performed on nonparametric distribution values26. These analyses were performed in GraphPad Prism program (GraphPad, version 7.0, USA). The significant level established was 5% (descriptive value of p < 0.05).
RESULTS
We observed a predominance of females in the negative COVID-19 group, corresponding to 61,5% of total patients. Male predominance occurred in the positive COVID-19 group with 63% of total infected patients. The mean age in the negative COVID-19 group was 44 ± 18 years, while the positive COVID-19 group presented a significantly higher mean, of 52 ± 19 years.
In positive COVID-19 patients lower values of erythrocytes was observed in comparison to the negative COVID-19 group, both between men (positive: 4.5 ± 0.8 vs. negative: 4.9 ± 0.4, CI 95%, p < 0.05) and between women (positive: 4.1 ± 0.8 vs. negative: 4.4 ± 0.3, CI 95% p < 0.05) (figures 1A and B). Hemoglobin also presented lower values in COVID-19 patients both between men (positive: 13.4 ± 2.4 vs. negative: 14.8 ± 1, CI 95%, p < 0.05) and between women (positive: 11.7 ± 2.4 vs. negative: 13.3 ± 0.9 CI 95%, p < 0.05), as shown in figures 1C and 1D.
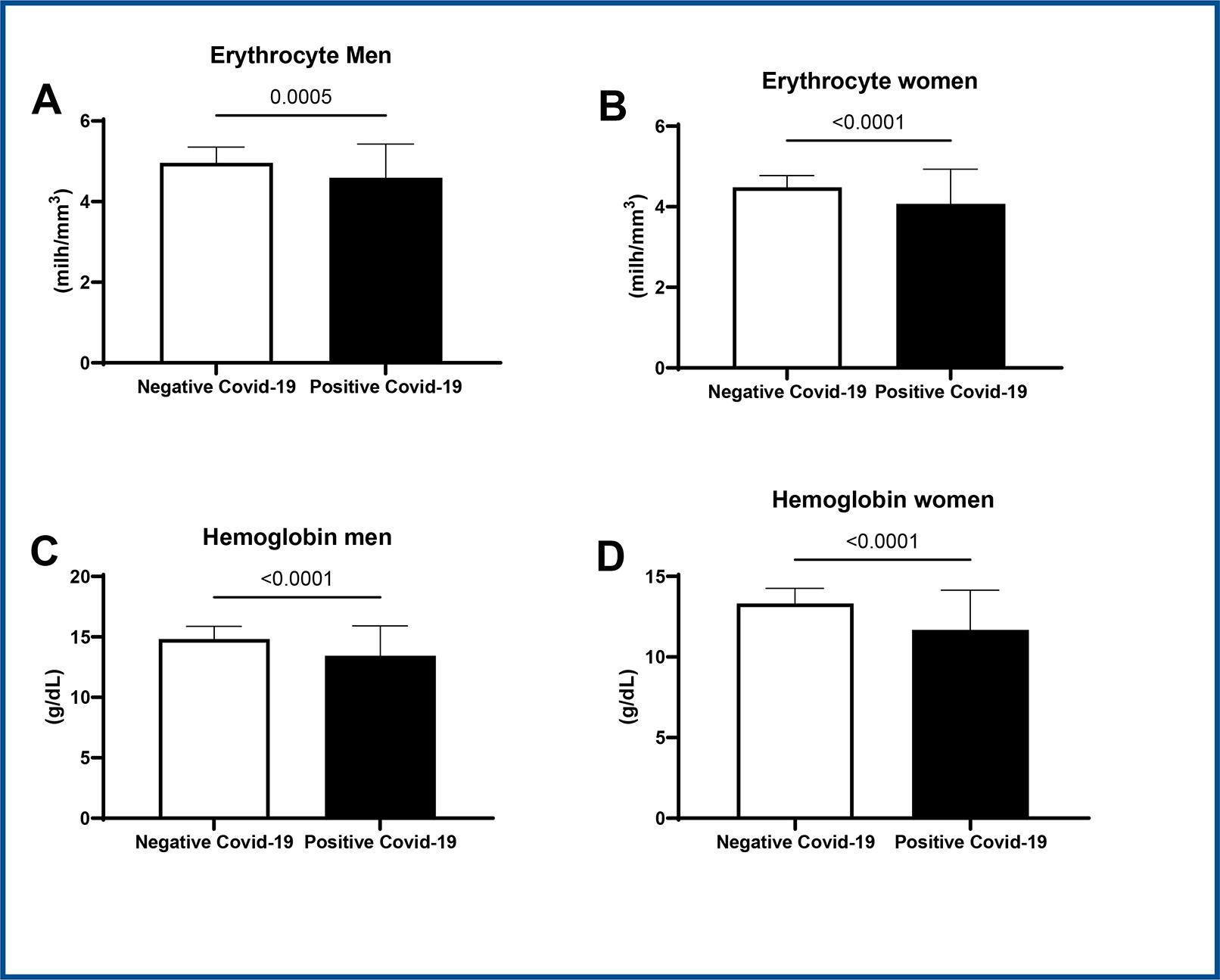
Student t-test * p < 0.05 vs. negative COVID-19, CI=95%. Data expressed as mean ± SD.
Figure 1 : Representative graph of erythrocytes values in men (1A) and women (1B). Hemoglobin in men (1C) and in women (1D) in COVID-19 positive patients versus COVID-19 negative patients.
Positive patients with COVID-19 also presented significantly lower values of hematocrit both between men (positive: 39.4 ± 6.7 vs. negative: 43.2 ± 2.9, CI 95%, p < 0.05) and between women (positive: 34. ± 7.1 vs. negative: 38.8 ± 3.5, CI 95%, p < 0.05) (figure 2A and B).
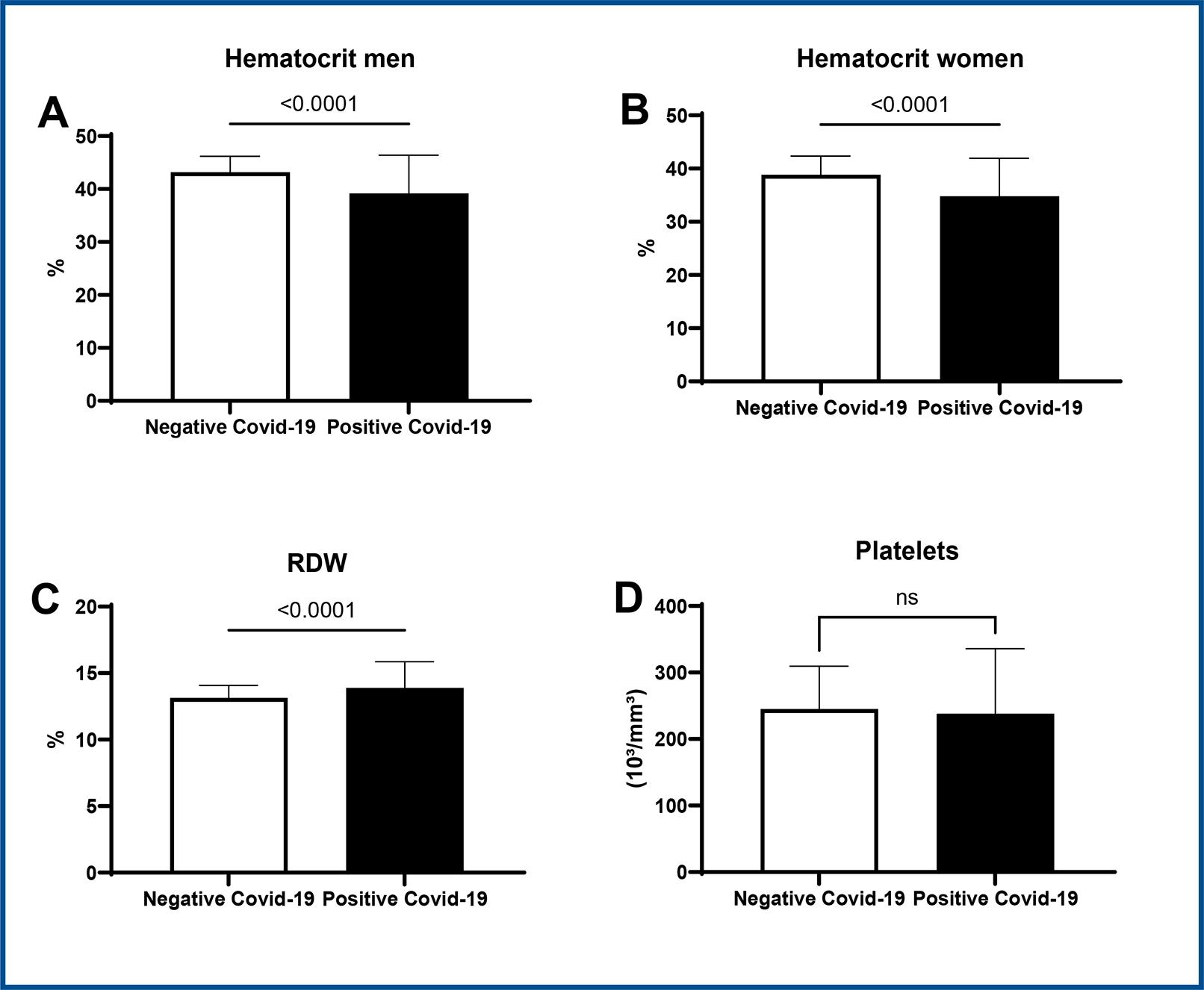
Student t-test * p < 0.05 vs. negative COVID-19, CI=95%. Data expressed as mean ± SD.
Figure 2 : Representative graph of hematocrit (2A and B), RDW (2C) and platelet (2D) values in COVID-19 positive patients versus COVID-19 negative patients.
RDW values were also higher in positive patients (positive: 13.89 ± 2.036 vs. negative: 13.133 ± 0.919, CI 95%, p < 0.05), as displayed in figure 2C. With regard to platelets, there was no statistical difference between groups (figure 2D).
Lower MCH values were observed in positive COVID-19 patients in comparison to negative patients (positive: 29.15 ± 2.74 vs. negative: 29.8 ± 1.73, CI 95%, p < 0.05) (figure 3B). There were no statistical differences in VCM or MCHC between groups (figure 3A and 3C).
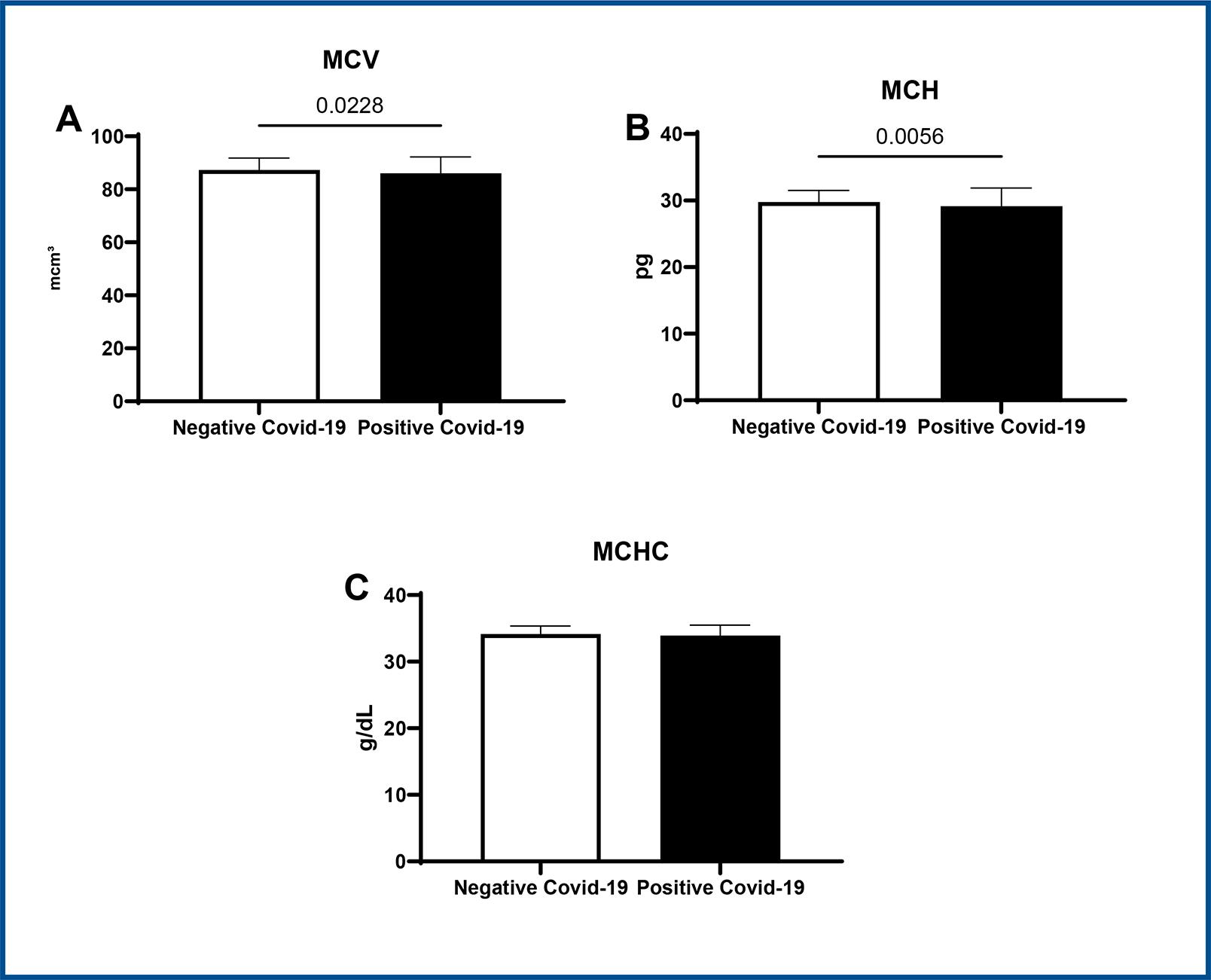
Student t-test * p < 0.05 vs. negative COVID-19, CI=95%. Data expressed as mean ± SD.
Figure 3 : Representative graphs of MCV (3A), MCH (3B) and MCHC (3C) values in COVID-19 positive patients versus COVID-19 negative patients.
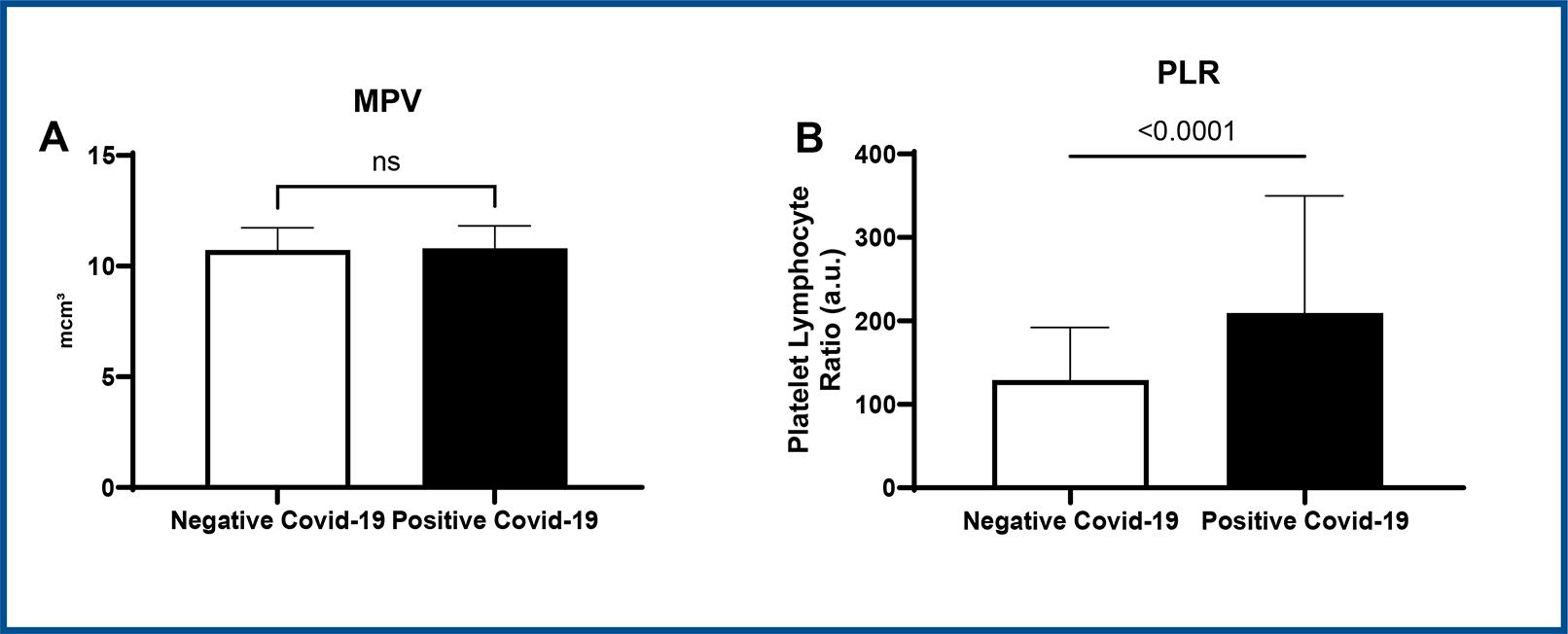
Student t-test * p < 0.05 vs. negative COVID-19, CI=95%. Data expressed as mean ± SD.
Figure 4 : Representative graphs of MPV values (A) and PLR values (B) in COVID-19 positive patients versus COVID-19 negative patients.
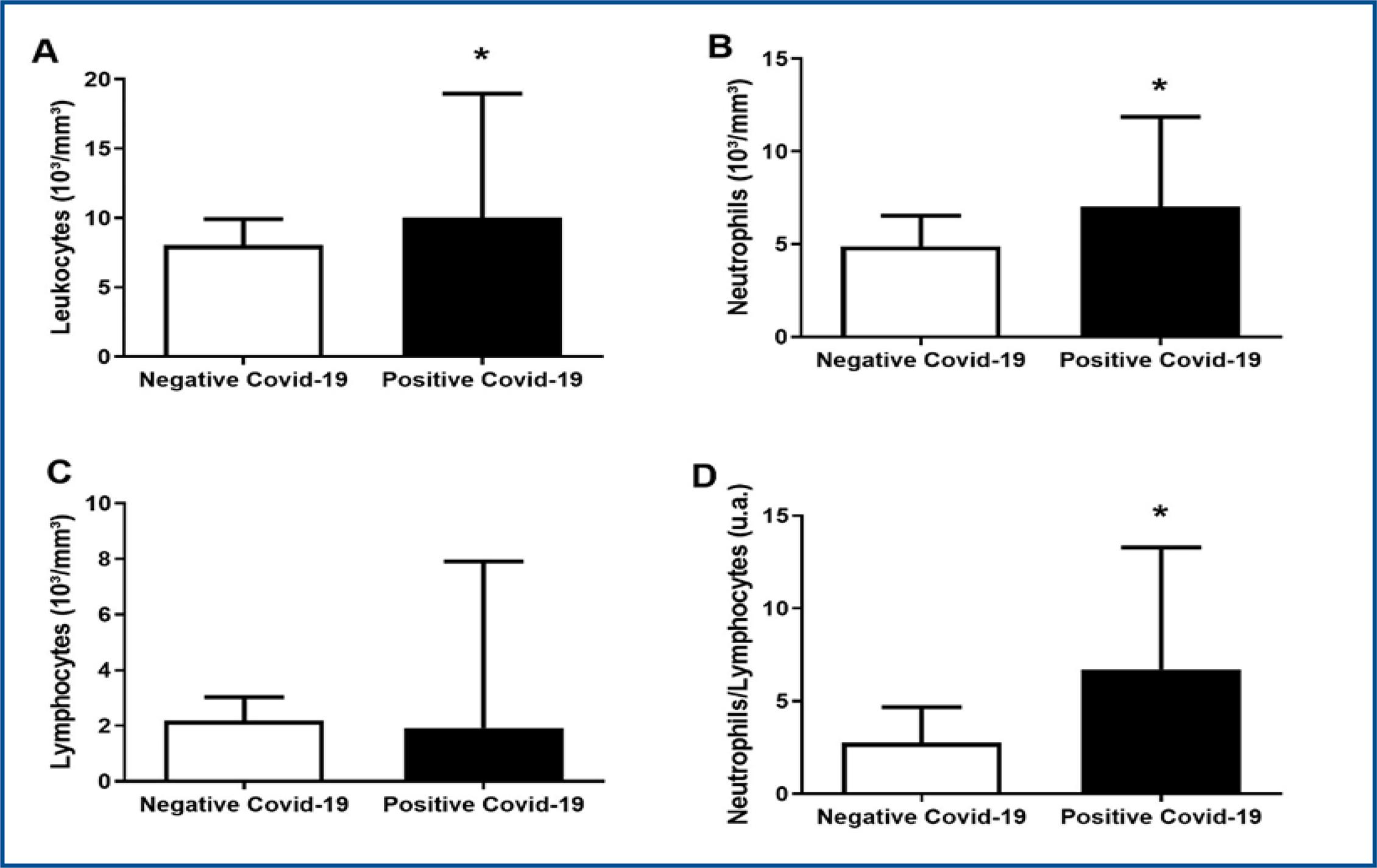
When compared to other hematological parameters, patients with COVID-19 presented higher leukocyte values (positive: 9.563 ± 5.633 vs. negative: 8.042 ± 1.877, CI 95%, p < 0.05) and neutrophil (positive: 7.028 ± 4.852 vs. negative: 4.892 ± 1.637, CI 95%, p < 0.05) as reported in figures 5A and 5B. Regarding lymphocyte values, no statistical difference between studied groups was found (figure 5C). The neutrophil/lymphocyte ratio was also higher in positive patients (positive: 6.738 ± 6.558 vs. negative: 2.758 ± 1.906, CI 95%, p < 0.05), as depicted in figure 5D.
DISCUSSION
The present study evaluated hematological variables in patients diagnosed with COVID-19 and in uninfected patients, and all samples originated from the public health system. The period of collections was before the start of vaccinations. In these circumstances we observed significant lower values for erythrocytes in infected patients, which is associated with abnormalities in erythrocyte structure due to codification of some non-structural proteins by viral RNA.
Moreover, in advanced stages of the disease an elevation was already observed in macrophage activity stimulated by cytokines, being equally harmful to erythrocytes. Furthermore, we observed a decrease in hemoglobin in which there are also structural impacts with removal of the iron atom caused by viral protein synthesis, impairing oxygen transport2. As expected, this alteration was reflected in hematocrit values which were significantly lower in positive COVID-19 patients as well. Progressive decrease in erythrocytes and hemoglobin may indicate worsening of the clinical course of the disease. Besides, significantly lower values of MCH were found in infected patients, indicating hypochromic anemia, due to low concentration of hemoglobin per red blood cell10.
Our study also observed higher values of RDW in positive COVID-19 patients, in concordance with as described by Foy et al. in a prospective study, where elevated RDW at hospital admission and increasing RDW during hospitalization were associated with higher mortality risk in these patients16. Hypoxia is believed to be one of the possible causes of the rise in this parameter since in order to compensate lack of oxygen there is an increase in erythropoietin and consequently in RDW, through mechanisms involving erythrocyte regulation of maturation and survival processes. This data reinforces that the increase in RDW may have an important role in the early detection of hypoxemia in positive COVID-19 patients; hence it is suggested that this parameter be closely monitored in these patients27.
In this study we observed elevated neutrophil values in positive patients. This increase is known to be a consequence of the cytokine storm caused by a hyperinflammatory state in SARS-CoV-2 infection, in addition to also being an indicator of concomitant bacterial infection. Likewise, we have obtained significantly lower values of leukocytes in positive COVID-19 patients which can be indicative of associated bacterial infection as well11.
Lymphopenia is a common finding in patients infected by SARS-CoV-2, probably because of an alteration in immune response due to viral infection28. Even though, in this study there was no statistical difference in lymphocyte counts between the groups. It was verified that lymphocyte values may vary according to disease course time11. Therefore, lymphocyte variability may possibly be associated with time of collection, since in this study the results were not related to disease progression.
We also evaluated neutrophil / lymphocyte ratio (NLR) which was significantly higher in positive COVID-19 patients, corroborating with the fact that NLR is already identified as a predictive and prognostic indicator of COVID-19 severity13.
Regarding platelet counts, there was no difference between the studied groups. Some studies describe lower platelet counts associated with higher mortality; however, many severe positive COVID-19 patients did not present this alteration. Despite this, as platelets have active participation in immune response, changes in their numbers and activity should be carefully analyzed29.
The MPV is an important parameter that has been studied in various clinical contexts, including infectious processes. Assessing MPV in evaluations of infectious processes can provide valuable insights into the body’s response to infection and inflammation. Nordin et al., (2004)24 conducted a multicentre study of reference intervals for haemoglobin, basic blood cell counts, and erythrocyte indices in the adult population of the Nordic countries, providing non-parametric reference intervals for B-Haemoglobin, B-Erythrocytes, B-EVF, B-MCV, Erc-MCH, and other hematological parameters. These reference intervals serve as valuable benchmarks for assessing hematological parameters, including MPV, in the context of infectious processes. Korniluk et al., (2019)30 discussed the new perspectives for an old marker in the course and prognosis of inflammation, emphasizing the role of MPV as a biomarker in various inflammatory conditions, including infectious diseases, diabetes mellitus, myocardial infarction, and cancer. In summary, the assessment of MPV in the context of infectious processes provides valuable information about platelet activity and the body’s response to infection, contributing to a better understanding of the hematological changes associated with infectious diseases.
The PLR has been extensively studied as an inflammatory marker and a potential indicator of disease activity in various medical conditions. Kim et al. (2015)31assessed the utility of PLR in patients with psoriasis vulgaris and psoriatic arthritis, highlighting its potential as an inflammatory marker in these conditions. Similarly, Qin et al. (2016)32 demonstrated the usefulness of PLR as a marker for assessing inflammatory response and disease activity in patients with systemic lupus erythematosus (SLE).
The correlation between PLR and COVID-19 infection has been examined. Sarkar et al. (2021)33conducted a systematic review and meta-analysis to assess the link between PLR at admission and outcomes, including mortality and severity among COVID-19 patients, offering valuable insights into the prognostic significance of PLR in COVID-19. Erdogan et al. (2021)34 investigated the prognostic role of PLR in COVID-19 patients, contributing insights into the utility of PLR as a prognostic indicator in this patient population. Aksu et al. (2021)35 explored the predictive role of PLR in assessing both lung involvement and severity in patients with COVID-19, providing valuable insights into the potential clinical applicability of PLR in evaluating COVID-19 severity.
The evaluation of PLR obtained by this study showed significantly higher values in positive COVID-19 patients compared to negative ones. Thus, PLR could be considered an important biomarker for prognostic estimations in SARS-CoV-2 infection, considering this parameter is associated with systemic inflammatory response. Nevertheless, this data needs further investigation concerning comorbidities. Additionally, new studies are needed to assess the applicability of this marker in the development of comorbidities post SARS-CoV-2 infection21.
Epidemiological studies have highlighted a link between SARS-CoV-2 infection and an increased risk of conditions such as cardiovascular diseases, diabetes, neurological disorders, and psychiatric disorders. Understanding these complex interactions is crucial for mitigating the long-term adverse effects of the disease.
Moreover, it is crucial to consider the role of individual patient characteristics, such as age, sex, pre-existing conditions, and immune response, in susceptibility to and manifestation of COVID-19-associated comorbidities. A thorough understanding of these alterations is essential to inform decision-making regarding appropriate disease control and management measures35-38.
In this study comparing the blood profile of positive versus negative COVID-19 patients, infection severity was not considered, also, the samples were not collected at a specific time of the disease course, these being the biases of this research. Among the study limitations, the lack of discrimination regarding the severity of the disease stands out. It is acknowledged that randomization based on the level of infection involvement could provide more accurate information on the evaluated indices and biomarkers. At first, the objective was mapping the major hematological alterations, before discriminating the severity of the disease, willing to describe a panel of this disease.
CONCLUSION
This study evidenced alterations in hematological parameters of positive COVID-19 patients. These data showed that the hemogram, a low-cost and minimally invasive exam, can support for screening, diagnosis and even prognosis of COVID-19, allowing better evaluation of the disease course and assisting medical decisions facing lack of resources in the pandemic setting.