Around 36,3 million people worldwide suffer from substance use disorders (SUD) ( United Nations Office on Drugs and Crime [UNODC], 2019). SUD is a chronic and multistage disease, which often debilitates the person with addiction, resulting in several adverse outcomes, for example, mental and physical health impairments, an increase in mortality rates, loss of academic and professional achievement, social impairments, and diminished global quality of life. This chronic nature and its multifactorial phenomena, comprising a variety of psychosocial factors (Beraldo et al., 2019; Crist et al., 2019), lead to challenging care. High rates of relapse, as well as low treatment adherence, are prevalent among persons with SUD (Baurley et al., 2022), corroborating rehospitalizations (Ilgen et al., 2008). It’s worth noting that rehospitalization in the context of SUD refers to the readmission of an individual to a healthcare facility for further treatment or intervention, often due to relapse or poor adherence to initial treatment plans (Nordeck et al., 2018). This study aims to identify and examine the risk and protective factors associated with this phenomenon of rehospitalization.
The low adherence to withdrawal treatments, in conjunction with the small provision of public health services directed for this population in many countries, results in low rates of SUD persons receiving proper therapies (Di Giovanni et al., 2020). Estimates suggest that only 14% of this population can manage withdrawal (UNODC, 2019). These rates are influenced by acute clinical symptoms such as craving, mood, and anxiety and contribute to at-risk for relapse (McHugh et al., 2014; Namba et al., 2018; Preston et al., 2018). Despite lapses and relapses being part of the process in SUD treatments, both represent fundamental challenges for clinicians in the withdrawal management of a person with addiction and could influence the course of rehospitalization (Franke et al., 2020), enhancing protective factors such as adherence to treatment (Shaffer et al., 2015; Walley et al., 2012) and social support of the patients (Benda, 2005) could be a step on this direction. Improving the SUD treatment cares during and post-discharge could be a way to reduce public health costs (Moreira et al., 2015) and increase the quality of life of the person with an addiction (Manthey et al., 2021).
A number of these rehospitalizations and failures in SUD treatment programs are recognized to be due to additional factors that are not necessarily caused by substance use or by the withdrawal symptoms experienced by the person in the absence of the drug (Crummy et al., 2020). The individual and environmental contexts, such as personal resources (Silva et al., 2021) and family and community support (Tractenberg et al., 2022), have already been linked to risk for rehospitalization (Ilgen et al., 2008; Min et al., 2007; Nijhawan et al., 2019). Early life adverse experiences refers to the experience of traumatic events during development in the form of specific variants such as sexual abuse, physical abuse, emotional abuse, physical neglect and emotional neglect, or overall childhood maltreatment (Bernstein et al., 2003) – and have been indicated as an essential factor that could influence the course of SUD treatment (Francke et al., 2013; Levandowski et al., 2016). Such experiences not only contribute to the complexity of the disorder but also create vulnerabilities that may alter treatment responsiveness (Tubman et al., 2021). Consequently, individuals with a history of early life adverse experiences may have a higher likelihood of poor treatment adherence (McLaughlin et al., 2020) and increased rates of rehospitalization in the post-treatment period (Andersson et al., 2019).
Considering the challenges of the person with SUD to maintain abstinence for long periods (Panlilio et al., 2020) and the range of clinical and psychosocial factors that could interplay in the engagement to treatment and response, it is important to identify and elucidate potential variables associated with relapse and rehospitalization. This would represent a significant first step toward future outcomes after discharge. For this reason, this systematic review aims to explore and summarize the existing research focused on risk and protective factors for relapse and rehospitalization.
METHODS
To ensure transparent and comprehensive reporting of the review process and findings, we adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (http://www.prisma-statement.org) (Moher et al., 2015) and the recommendations of Cochrane for developing a search strategy (Higgins et al., 2019) were followed. It was registered on the International prospective register of systematic reviews (PROSPERO) and can be found with the code CRD42021247937.
SEARCH STRATEGY AND ELIGIBILITY
The search was conducted on April 12th, 2021, in the following databases: PubMed, Web of Science, and Embase. The descriptors used for the search were: ((“substance related disorders) OR (“substance abuse”)) OR (“drug addiction”) AND (([“patients rehospitalization) OR “patients” “readmission” OR rehospitalization])). The search strategy was adapted for each database. Importantly, there was no time limit set on the search string, allowing for a comprehensive review of the literature from the inception of each database up to April 2021. An updated search with the same terms was conducted on January 28th, 2024, to include four new publications. This update was necessitated by the extended duration of the project, ensuring the inclusion of the most recent and relevant studies.
Cross-sectional, cohort, and case-control studies that evaluated persons with SUD who have relapse or rehospitalization as a followed outcome were considered eligible for this review. The exclusion criteria were: (a) studies that were not published in peer-reviewed journals; (b) preclinical studies; (c) articles published in other languages that are not English.
SELECTION OF STUDIES AND DATA EXTRACTION
Duplicates were excluded before the screening of the studies. Titles and abstracts were then screened independently by three authors (JBT, RAM, and BPM) to verify if they fulfilled the eligibility. The initial screening conflicts were resolved by a senior author (SGT). After selecting the articles based on titles and abstracts, the full texts were read to decide which articles would be included in the systematic review.
The following data were then extracted from all included studies by the three independent authors that first screened the articles: “first author,” “publication year,” “sample size,” “study design,” “intervention,” “treatment,” “follow-up” and “quantitative and qualitative main findings.”
METHODOLOGICAL QUALITY AND RISK OF BIAS ASSESSMENT
To assess the methodological quality and risk of bias of the included studies, an adapted version of the Newcastle-Ottawa Quality Assessment Form for Cohort Studies was used (Stang, 2010). This 8-point scale is divided into three categories: selection, comparability, and outcome. Each category has criteria corresponding to 1 point, except the comparison category in which it is possible to assign two stars if the study has controlled for more than one factor. The selection category was adapted and comprised three criteria: representativeness of the sample, selection of the comparison cohort, and ascertainment of exposure. The comparability category had 1 criterion: comparability of samples based on the design or analysis. Finally, the outcome category had three criteria: assessment of outcome, long enough follow-up for outcomes to occur, and adequacy of follow-up of cohorts. Studies with a total of 7 points or higher were considered low risk of bias.
RESULTS
SEARCH RESULTS
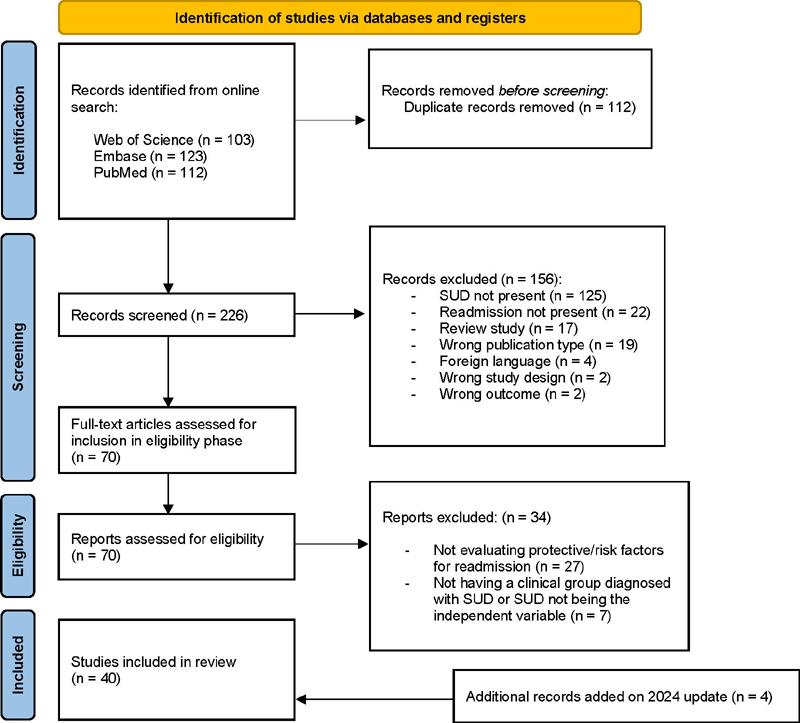
The initial search yielded 338 studies. After duplicates were removed (n = 112), we screened 226 studies by reviewing the title and abstract. 156 studies were excluded, and the remaining studies (n = 70) were full text reviewed. Following the application of the exclusion criteria, a total of 40 studies were included in the review. Detailed information about the inclusion process can be found in the flowchart (Figure 1).
CHARACTERISTICS OF THE STUDIES
Data from a total of 268,378 persons diagnosed with SUD were analysed. The range of publication years was between 1982–2023. For each article, we included characteristics regarding the sample sizes, preference of substance, mean age of SUD group, study design, and type of treatment (see Table 1) - if it had any interventions, time of follow-up, and a summary of the results. Of the n = 40 studies fully analyzed, 45.0% (n = 18) had a longitudinal retrospective design; another 37.5% (n = 15) had a prospective longitudinal design; 7.5% (n = 3) were randomized controlled trials; 2.5% (n = 1) had a prospective cohort design; 2.5% (n = 1) were non-randomized controlled trials; 2.5% (n = 1) were quasi-experimental trials; and 2.5% (n = 1) did not report the study design. Less than half of the studies included interventions (37.5%; n = 15). The interventions presented in the studies are explained in (Table 2). The time of follow-up varied from 4 to 1095 weeks.
Table 1 Characteristics of the included studies.
| Authors (Year) | Substance | Sample size | SUD Age (mean, SD) | Women, % | Study design | Intervention (yes/no) |
|---|---|---|---|---|---|---|
| Ahacicet al. (2014) | Alcohol | 54955 | NR | NR | Longitudinal retrospective | No |
| Benda (2002) | Polysubstance | 600 | 51 (1.67) | NR | Longitudinal retrospective | Yes |
| Benda (2004) | Polysubstance | 625 | 36.5 (8.3) (W)/ 45.9 (10.6) (M) | 49.6 | Longitudinal prospective | Yes |
| Brennan et al. (2000) | Polysubstance | 22768 | 72.9 | 37.0 | Longitudinal prospective | No |
| Decker et al. (2017) | Polysubstance | 207 | 50 (6.1) | 9.0 | Longitudinal retrospective | Yes |
| D’Ercole et al. (1997) | Polysubstance | 289 | 34.7 (12.1) | 59.0 | Randomized control trial | Yes |
| Di Lorenzo et al. (2016) | Polysubstance | 105 | 40.2 (13.5) | 45.0 | Longitudinal retrospective | No |
| Dixon et al. (2009) | Polysubstance | 135 | 47.77 (7.81) | 0.0 | Randomized control trial | Yes |
| Dixon et al. (1997) | Polysubstance | 168 | 32.7 (9.3) | 43.0 | Longitudinal prospective | No |
| El-Mallakh et al. (2004) | Polysubstance | 81 | 34.6 (11.9) | 65.4 | Longitudinal retrospective | No |
| Erfan et al. (2010) | Polysubstance | 60 | 28.1 (5.0) | 0.0 | Longitudinal retrospective | No |
| Farren e McElroy (2010) | Alcohol | 183 | 43.2 (13.7) | 50.27 | Longitudinal prospective | Yes |
| Franke I. et al. (2020) | Polysubstance | 501 | 33.19 (9.6) (W)/ 33.58 (8.59) (M) | 22.16 | Longitudinal retrospective | Yes |
| Hellerstein et al. (1995) | Polysubstance | 47 | 31.9 (6.7) | 23.4 | Longitudinal prospective | Yes |
| Ilgen et al. (2008) | Polysubstance | 26826 | 50.0 (9.0) | 21.7 | Longitudinal prospective | No |
| Irmiter et al. (2007) | Polysubstance | 22230 | 47(10) | 6 | Longitudinal retrospective | No |
| Irmiter et al. (2009) | Polysubstance | 250 | NR | 46 | Longitudinal retrospective | No |
| Kartha et al. (2007) | Polysubstance | 144 | 55 (16.3) Rehospitalized (n=64) // 54.6 (15.4) Not rehospitalized (n=80) | 71 and 64 | Prospective cohort | No |
| Kelly et al. (2003) | Polysubstance | 45 | 31.92 (5.68) / 35.86 (8.44) | 21,05 // 53.85 | Non-randomized controlled trial | Yes |
| Leon et al. (1998) | Polysubstance | 163 | 35.8 (10.8) | 63.2 | Longitudinal prospective | No |
| Lin et al. (2014) | Methamphetamine | 756 | 34.1 (6.2) / 37.7 (10.6) | 27.0 // 46.0 | Longitudinal retrospective | No |
| Luo et al. (2022) | Opioid | 1703 | NR | 33.7 (control) // 30.2 (C.L.I.M.B) | Quasi-experimental trial | Yes |
| Maturana et al. (2023) | Polysubstance | 85048 | 34 (14-88) ambulatory / 33 (15-80) residential* | 25.2 // 33.2 | Longitudinal retrospective | No |
| Miner et al. (1997) | Polysubstance | 49 | 32.08 (6.62) | 76.0 | Longitudinal prospective | Yes |
| Min et al. (2007) | Polysubstance | 484 | 36.8 (7.7) /37.9(9.6) | 35.0/35.0 | Longitudinal prospective | Yes |
| Moggi et al. (2002) | Polysubstance | 84 | 31.7 (7.78) | 33.3 | Longitudinal prospective | Yes |
| Moos et al. (1994) | Polysubstance | 16066 | 62.0 (NR) | 2.0 | Longitudinal retrospective | No |
| Moos et al. (1995) | Polysubstance | 10352 | 42.0 (NR) | 1.0 | Longitudinal retrospective | No |
| Nordeck et al. (2018) | Polysubstance | 267 | 48.6 (11.6) | 42.3 | Longitudinal retrospective | No |
| O’Toole et al. (2007) | Polysubstance | 390 | 41 (35-48) / 41 (36-48) | 34.9 // 39.5 | Longitudinal prospective | Yes |
| Rabiee et al. (2023) | Cannabis | 12143 | 22.9 (9.5) no readmission // 22.9 (9.9) readmission* | 77.4 // 22.6 | Longitudinal retrospective | No |
| Romelsjö et al. (2005) | Polysubstance | 296 | 47.0 (W) / 49.0 (M) | 33.78 | Longitudinal prospective | No |
| Russolillo et al. (2023) | Polysubstance | 3907 | 40.66 (14.33) | 37.73 | Longitudinal retrospective | No |
| Shaffer et al. (2015) | Polysubstance | 373 | 39.7 (11.8) | 43.0 | Randomized control trial | Yes |
| Slater e Linn (1982) | Alcohol | 200 | NR | 0.0 | Longitudinal prospective | No |
| Swofford et al. (2000) | Polysubstance | 262 | 42.2 (10.94) | 44.0 | Longitudinal retrospective | No |
| Viola et al. (2014) | Polysubstance | 93 | 27.45 (6.4) / 30.32 (6.4) | 100 | Longitudinal prospective | No |
| Walker et al. (1994) | Polysubstance | 3087 | 42.7 (10.4) | NR | NR | No |
| Walker et al. (1995) | Polysubstance | 1698 | 39.9 (12.6) / 53.9 (18.2) | 100 | Longitudinal retrospective | No |
| Walley et al. (2012) | Polysubstance | 738 | 47.8 (11.4) / 50.2(5.8) | 35.0 /53.0 | Longitudinal prospective | No |
Note: SUD, substance use disorder; SD, standard deviation; NR, not reported; W, women; M, men; *, only median reported.
Table 2 Intervention descriptions.
| Authors (Year) | Treatment | Treatment Description |
|---|---|---|
| Benda (2002) | Detoxification; Drug and mental health treatment | The program consists of a 30-day inpatient detoxification intervention in which patients are prohibited to use any substance as well as a program aimed helping them to find and keep a job and achieve an independent life. |
| Benda (2004) | Domiciliary program | The program included, but was not limited to, cognitive-behavioural and insight therapies for individuals and groups, the 12-step AA and NA approaches, preparation for and obtaining employment, independent living skills, and establishing social supports. |
| Decker et al. (2017) | Mental Health Residential Recovery Program (MHRRTP) | The program consists of 60 days of intensive mandatory psychoeducational; focal group, and individual intervention; participants are required to attend three to four Alcoholics Anonymous (AA) or Narcotics Anonymous (NA) per week, the 12-step AA and NA approaches; random urine test; help participants find housing and employment. |
| D’Ercole et al. (1997) | Case management intervention - Protocol based on The Case Management Activity Form (CMAF) | A protocol consists of activities that fit into ten categories: direct services, including assessment, advocacy, and implementation of services; supervision and staff coordination; escorting patients to programs, hospitals, entitlement agencies, and the like; administrative activities and paperwork; family-related activities such as discussing the patient’s problems with the family or supporting the family in some way; visiting the patient in the community or the hospital; medical and health-related activities; medication monitoring; patient and family education and therapy. |
| Dixon et al. (2009) | Brief three-month critical time intervention (B-CTI) | The intervention covers nine target areas such as systems coordination; engagement in psychiatric services; continuation of substance abuse treatment; medication adherence; family involvement and social support network; life skills training and support; integration of medical care; establishment of community linkages; practical needs assistance. |
| Farren e McElroy (2010) | FIRESIDE program | The program consists of three stages: detoxification and mood stabilization; a program with a cognitive-behavioral, relapse prevention approach to both affective and substance use disorder; aftercare program for up to 6 months post-discharge. |
| Franke et al. (2020) | Hospital program | The hospital in Taufkirchen provides treatment for female offenders only. The participating hospitals have different kinds of wards, ranging from high to low-security levels, and patients are transferred from higher- to lower-security units according to their therapeutic progress. |
| Hellerstein et al (1995) | Integrated treatment vs. standard treatment (control) | Integrated treatment consists of outpatient supportive psychotherapy and psychoeducation twice per week and psychopharmacological management. Standard treatment consists of comparable levels of substance abuse and psychiatric service (predominantly case management, group psychotherapy, and psychopharmacology). |
| Kelly et al. (2003) | Pharmacological treatment | Clozapine treatment. |
| Luo et al. (2022) | Community-based Life-changing Individualized Medically assisted evidence-Based treatment [C.L.I.M.B.] | The C.L.I.M.B. pilot program is a community-based treatment model for substance use disorders, integrating a comprehensive continuum of care. Services span from detoxification, residential, partial hospitalization/ intensive outpatient, to standard outpatient services, alongside Medication for Opioid Use Disorder (MOUD). The program uniquely incorporates a modified smartphone application, A-CHESS, based on the self-determination theory, to support patients’ recovery journey. A-CHESS, originally developed at the University of Wisconsin, serves as a holistic tool in aiding individuals to maintain sobriety and succeed in recovery. |
| Miner et al. (1997) | Experimental treatment vs. standard treatment (control) | Patients were randomized to 2 mode-of-service conditions, one providing experimental integrated psychiatric and substance abuse treatment and the other providing standard care as a control. Also, patients in both conditions were counseled about their aftercare and accompanied by staff to their first group therapy session before discharge from the inpatient service. |
| Min et al. (2007) | The Friends Connection Program (FC) | Assist participants in developing skills necessary for living a satisfying and fulfilling life in the community without drugs and alcohol. The program also attempts to enhance the client’s social network and social support with new people who do not use drugs or alcohol. |
| Moggi et al. (2002) | Integrated Inpatient Treatment | The program comprises four treatment stages: engagement, motivation, intensive treatment, and transition. |
| O’Toole et al. (2007) | Structured day hospital intervention integrating substance abuse treatment and medical care | Treatment consists of individual and group cognitive-behavioural therapy, primary and specialty medical care, occupational therapy, physical therapy, and other necessary medical and mental health services associated with their acute illness. |
| Shaffer et al. (2015) | Brief critical time intervention (BCTI) | Evidence-based practices are highly effective in helping individuals with serious mental illness to connect with community resources, begin outpatient treatment after hospital discharge, and find emotional and practical support during transition periods. BCTI is a three-month version of CTI (critical time intervention) that is used as an individual-level intervention intended to prevent recurrent homelessness among persons with serious mental illness during periods of transition. |
According to the NOS table (see Table 3, supplementary materials), a significant portion of the studies assessed, 25 studies (62.5%), scored below 7 and were categorized as “high risk of bias.” Conversely, 15 studies (37.5%) achieved a score of 7 or higher, indicating a “low risk of bias.” Notably, several studies did not include detailed information about their sample in the methods section. Instead, they referenced other studies for adequate sample information or relied on the same sample group for their analysis.
Table 3 Modified Newcastle–Ottawa Scale (NOS) for assessing the quality of studies.
| Studies | Selection | Comparability | Outcome | TOTAL | ||||
|---|---|---|---|---|---|---|---|---|
| Reference | Representativeness of the sample | Selection of the comparison cohort | Ascertainment of exposure | Comparability of samples on the basis of the design or analysis | Assessment of outcome | Was follow-up long enough for outcomes to occur? | Adequacy of follow up of cohorts | |
| Ahacic et al. (2014) | * | * | * | ** | * | * | - | 7 |
| Benda (2002) | * | - | * | ** | * | * | - | 6 |
| Benda (2004) | * | - | - | ** | * | * | - | 5 |
| Brennan et al. (2000) | * | * | - | ** | * | * | - | 6 |
| Decker et al. (2017) | * | * | - | ** | * | * | - | 6 |
| D’Ercole et al. (1997) | * | * | * | ** | * | * | * | 8 |
| Di Lorenzo et al. (2016) | * | * | - | ** | * | - | - | 5 |
| Dixon et al. (2009) | * | * | * | ** | * | * | - | 8 |
| Dixon et al. (1997) | * | * | - | - | * | * | - | 4 |
| El-Mallakh et al. (2004) | * | - | * | * | * | - | * | 5 |
| Erfan et al. (2010) | * | * | * | - | * | * | - | 5 |
| Farren e McElroy (2010) | * | - | * | ** | * | * | - | 5 |
| Franke et al. (2020) | * | * | - | * | * | * | - | 5 |
| Hellerstein et al. (1995) | * | - | * | ** | * | * | - | 6 |
| Ilgen et al. (2008) | * | * | * | ** | * | * | - | 7 |
| Irmiter et al. (2007) | * | - | * | * | * | * | - | 5 |
| Irmiter et al. (2009) | * | * | * | * | * | * | * | 7 |
| Kartha et al. (2007) | * | * | * | ** | * | * | - | 7 |
| Kelly et al. (2003) | * | * | - | - | - | * | * | 4 |
| Leon et al. (1998) | * | * | * | * | * | * | - | 6 |
| Lin et al. (2014) | * | - | * | * | * | * | * | 6 |
| Luo et al. (2022) | * | * | * | ** | * | * | * | 8 |
| Maturana et al. (2023) | * | * | * | ** | * | * | * | 8 |
| Miner et al. (1997) | * | * | * | * | - | - | - | 4 |
| Min et al. (2007) | - | * | - | * | * | * | - | 4 |
| Moggi et al. (2002) | * | - | - | * | * | * | - | 4 |
| Moos et al. (1994) | * | - | * | ** | * | * | * | 7 |
| Moos et al. (1995) | * | * | * | ** | * | * | - | 7 |
| Nordeck et al. (2018) | * | - | * | ** | * | * | * | 7 |
| O’Toole et al. (2007) | - | * | * | - | * | * | - | 4 |
| Rabiee et al. (2023) | * | * | * | ** | * | * | - | 7 |
| Romelsjö et al. (2005) | * | - | * | ** | * | * | - | 6 |
| Russolillo et al. (2023) | * | - | * | ** | * | * | - | 6 |
| Shaffer et al. (2015) | * | * | - | ** | * | * | - | 6 |
| Slater e Linn (1982) | * | - | - | - | * | * | - | 3 |
| Swofford et al. (2000) | * | * | * | ** | * | * | - | 7 |
| Viola et al. (2014) | * | - | * | ** | * | * | * | 7 |
| Walker et al. (1994) | * | * | * | * | * | - | - | 5 |
| Walker et al. (1995) | * | * | * | ** | * | - | - | 6 |
| Walley et al. (2012) | * | * | * | ** | * | * | * | 8 |
MAIN FINDINGS OF INCLUDED STUDIES
The studies included described multiple potential risk and a few protective factors for relapse and rehospitalization. Considering the heterogeneity of variables reported by the studies as risk or protective for SUD treatment outcomes, and rehospitalization, we decided to perform a qualitative analysis based on the description of the results. We took into account the main idea that those variables were intended to be pointed out and grouped them into categories, as follows: for risk factors, ‘Comorbidities’; ‘Trauma’; ‘Treatments or outpatient follow-up dropout’; ‘Lack of adherence to treatment programs’; ‘History of hospitalization’; ‘History and patterns of drug use’; ‘Family and Social problems’; ‘Occupational Status’; ‘Sex’; ‘Medical Condition’; ‘Age’; ‘ethnicity’; and ‘Housing condition’. For protective factors, ‘Adherence to treatment programs’; ‘Social and familial support’; ‘Self-efficacy and self-characteristics’; ‘Age’; ‘Religiosity’; and ‘Income and employment status. These findings are presented in a percentage table representing the different categories (Table 4). The most prevalent finding for risk factors were the treatment dropouts and the presence of psychiatric comorbidity. Commitment to treatment and age were the most pervasive reported protective factors. Among the treatments and interventions reviewed, three explicitly incorporate cognitive-behavioural therapy elements: a domiciliary program, “the ‘FIRESIDE Program,” and the “Structured Day Hospital Intervention.” These findings are particularly relevant to the journal’s Cognitive and Behavioral Therapies focus.
Table 4 Frequency of citations in each of the different categories.
| Risk factors for relapse | N | % |
| Comorbidities | 28 | 30.43 |
| Trauma | 4 | 4.35 |
| Failed program | 14 | 15.22 |
| History of hospitalization | 12 | 13.04 |
| History and patterns of drug use | 6 | 6.52 |
| Family and social problems | 5 | 5.43 |
| Occupational status | 3 | 3.26 |
| Sex | 5 | 5.44 |
| Medical condition | 6 | 6.52 |
| Age and ethnicity | 4 | 4.35 |
| Housing | 5 | 5.44 |
| Protective factors | N | % |
| Adherence to treatment/specific programs | 27 | 44.26 |
| Social and familial support | 12 | 19.67 |
| Self-efficacy and characteristics of self | 10 | 16.39 |
| Age | 2 | 3.28 |
| Religiosity | 2 | 3.28 |
| Income and employment | 3 | 4.92 |
| Psychiatric condition | 5 | 8.20 |
Fonte: Criado pelos autores.
DISCUSSION
This study explored risk and protective factors on relapse and rehospitalization rates. Our systematic review and qualitative analysis of the reports from included studies indicated that there was sufficient evidence concerning multiple recurring factors, from pre to during and post-treatment influencing relapse or rehospitalization rates of a person with SUD. The main issues highlighted was the presence of psychiatric comorbidities; history of traumatic life experience; history of treatments dropouts; the number of previous rehospitalization; history and patterns of drug use; family and social problems; occupational status; sex; prior medical condition; age; ethnicity; and housing condition. The recurrence of studies reporting these factors as essential to be followed during treatments as potential risk factors for unsuccess in the SUD treatment outcomes highlighted the need for improvement and a more personalized approach in substance use health care programs. In addition, we found a disparity between studies exploring risk factors compared to protective factors. The latest has three main factors reported by the reviewed studies: adherence to treatment, social and familial support, self-efficacy, and characteristics of self. It suggests that most of the evidence available on protective factors for rehospitalization is based on individual motivation and self-efficacy, familiar and social support, going beyond the range of detoxification, and short-term intervention programs focusing on sustaining abstinence.
Numerous studies have highlighted psychiatric comorbidities as a significant risk factor for rehospitalization in individuals with Substance Use Disorders (SUD) (Benda, 2002; Brennan et al., 2000; Decker et al., 2017; Di Lorenzo et al., 2016; Erfan et al., 2010; Ilgen et al., 2008; Lin et al., 2014; Min et al., 2007; Moggi et al., 2002; Moos et al., 1994; Nordeck et al., 2018; O’Toole et al., 2007; Shaffer et al., 2015; Slater & Linn, 1982; Walley et al., 2012) Notably, almost 30% of the included studies indicated that individuals with psychiatric comorbidities experience higher rates of rehospitalization. Some of these studies, including those by Ilgen et al. (2008), Lin et al. (2014) and Shaffer et al. (2015), reported this trend within 90 days of discharge. Ilgen et al. (2008) observed that over 23% of comorbid patients were rehospitalized within a few months, while Decker et al. (2017) noted that 59% of their sample had a comorbid psychiatric disorder. Additionally, Rabiee et al. (2023) found that individuals diagnosed with schizophrenia and other psychotic disorders, mood-related disorders, or personality disorders had an elevated risk of readmission.
In Romelsjö et al. (2005), Decker et al. (2017), Min et al. (2007), Miner et al. (1997), Hellerstein et al. (1995), and Swofford et al. (2000) studies, it was suggested that some factors related to the individual course during the SUD treatment itself raise the likelihood of relapse and rehospitalization. The studies that had interventions reported that the lack of adherence to the proposed intervention and the lack of overall participation during treatment were predictors for relapse. Rehospitalization commitment of a person with SUD has been suggested as one of the main issues for intervention-focused treatments and most follow-up studies (Herbeck et al., 2005). Other studies (Romelsjö et al., 2005) (Hellerstein et al., 1995) that did not have interventions on their protocol, still presented a higher likelihood of rehospitalization for those patients that did not seek follow-up treatment after discharge. Another recurring risk factor was displayed by the patients that had prior hospitalization history: Brennan et al. (2000), Benda (2002), Moos et al. (1994, 1995), Ahacic et al. (2014), and Erfan et al. (2010) reported that in their sample, patients that had a prior history of hospitalization due to substance use ended up readmitting more during the study, in comparison with their non or less hospitalized counterparts (Ahacic et al., 2014). This occurrence is an essential aspect of being named the revolving door phenomenon in the literature, in which the user repeatedly falls back to relapse after rehospitalization and goes back to the system on a chronic cycle.
Drug addiction is well established as a chronic condition characterized by different stages: drug use, abstinence; withdrawal symptoms, including drug craving; lapse and relapse, and, finally, recurrence of use (Koob & Volkow, 2016). In this sense, it is crucial to recognize the patterns of substance use and how they could represent an individual characteristic that interferes with SUD withdrawal management and treatment (Nordeck et al., 2018; Stahler et al., 2009). In addition to the substance use trajectory, another issue referred to by the studies is related to polysubstance use, a condition many people with SUD engage in (Crummy et al., 2020; Erfan et al., 2010). The reviewed studies, for example, assessed different samples of people with addiction and suggested that those with a more chronic pattern of substance use and those with polysubstance users were among the high-risk group for relapse and rehospitalization (Dixon et al., 1997). Both chronicity and polysubstance use have been pointed to as significant predictors for relapse and rehospitalization, indicating that the use of more than one substance and the chronicity of addiction increase the overall risk and represent a challenge during withdrawal (Andersson et al., 2019; Crummy et al., 2020). In the study of Nordeck et al. (2018), polysubstance use disorders, such as simultaneous alcohol, opioid, and cocaine use, have been associated with an increased proportion of rehospitalized rates compared to a person with single use-related disorders.
Considering the less frequently reported factors at risk for a person with SUD, our findings elucidated that exposure to adverse life experiences, independently of diagnosis of post-traumatic stress disorder (PTSD), such as childhood maltreatment, domestic and sexual violence, or other kinds of traumatic experience, was a corollary for a higher rate of rehospitalization (Benda, 2005; Levandowski et al., 2016). In Benda’s sample, it was suggested that homelessness and lack of appropriate housing conditions significantly contribute to relapse and rehospitalization. In Russolillo et al. (2023) retrospective cohort of 3907 individuals (of which 686, or 17.56%, had no housing condition), homelessness at discharge was associated with increased 30-day and 90-day psychiatric readmission - the most notable risk factor of the study. Additional sociodemographic factors, such as age, sex, occupational status, marital status, and ethnicity, also were elucidated, and depict how not only symptoms but the personal characteristics could influence the treatments outcomes (Donisi et al., 2016; Irmiter et al., 2007; Moos et al., 1995; Rabiee et al., 2023; Stahler et al., 2009; Walley et al., 2012). Self-efficacy, a cornerstone in Relapse Prevention theory, was also reiterated as a crucial factor in the literature (Donisi et al., 2016). This reinforces the importance of incorporating well-established psychological constructs, such as self-efficacy, into personalized treatment protocols for SUD, alongside addressing the specific context of drug addiction.
In contrast to risk factors, those considered protective were found as the most recurrent reported for the studies contemplated six different categories, of which three were more frequent. The studies of Ilgen et al. (2008), Romelsjö et al. (2005), Decker et al. (2017), Min et al. (2007), Farren e McElroy (2010), Nordeck et al. (2018), Hellerstein et al. (1995), Shaffer et al. (2015), Walley et al. (2012), Dixon et al. (2005), D’ercole et al. (1997) and Walker et al. (1994) reported that the best predictor for fewer rehospitalizations was searching for treatment or maintenance of both inpatient and outpatient follow-up as well as adherence to a proposed program Specifically, Luo et al. (2022) demonstrated that participation in the community-based life-changing individualized medically assisted evidence-based treatment (C.L.I. M.B.) program was a protective factor against readmission for opioid use disorder, exemplifying the effectiveness of well-structured treatment programs. Treatment completion, as per the findings from the Chilean sample in the study by Maturana et al. (2023), is instrumental in mitigating readmission risks. The data reveal a substantial reduction in readmission likelihood, marked by a 17% decrease for the first event and a 14% decline for the second entry in ambulatory treatments. Another protective factor pointed out was family and social support, which also was reported as at-risk for a person with SUD in case of absence. Studies by Benda (2002, 2005), Slater e Linn (1982), and Di Lorenzo et al. (2016) reported how much of a difference a social support system makes in helping patients remain abstinent after discharge from SUD treatment. Having a supportive family and friends willing to help and empower the patient post-discharge enhances treatment adherence, a clear highlight of these results – similar evidence can be found in the rehospitalization literature (Braet et al., 2016). In Benda (2002, 2005) publications, the importance of the connection with relatives and their parental care capability was a promoter of self-efficacy and maintenance of abstinence. Out of the treatment methods analyzed, three explicitly incorporate elements of cognitive-behavioural therapy: the “Domiciliary Program,” the “FIRESIDE Program,” and the “Structured Day Hospital Intervention Integrating Substance Abuse Treatment and Medical Care.” These types of treatments may be particularly effective in managing Substance Use Disorders. Given the journal’s specific interest, it is noteworthy that only a limited number of the treatments under study employ cognitive-behavioural techniques. This could indicate a gap in current treatments and present an avenue for further research.
FINAL CONSIDERATIONS
In this exploratory and qualitative systematic review approach, we could provide some directions to clinicians planning SUD treatments. However, we believe some limitations should be discussed in our findings. Firstly, most risk factors for rehospitalization elucidated in this review, such as psychiatric comorbidity, psychological trauma, and polysubstance use, are also associated with the severity and progression of the clinical course of SUD itself. This interplay should be considered regarding the clinical implications of our findings, as higher rehospitalization rates in people with SUD are also related to the severity of the disease (Jing et al., 2020). We found plenty of heterogeneity in sample characteristics and methodological aspects underlying the assessment of relapses and rehospitalization. Even though the sample sizes in most of the reviewed studies are not small, most of the investigated samples are very specific (i.e., war veterans, rural area citizens). It represented a challenge to summarize the results and achieve our main goals in this review. For this reason, we adopted a more qualitative approach in our analyses, categorizing the descriptions of the main findings and later applying quantitative summarization. Because we did not restrict eligibility of the included studies based on design, outcomes, and statistics, we did not aim to perform quantitative analyses. Hence, future studies aiming at pooling statistical data and performing a meta-analysis might further clarify the most relevant risk and protective factors that influence rehospitalization rates in SUD. Additionally, future studies should focus on more representative populations and those addressed by some of the included studies, allowing for greater power of comparison. Another essential factor to point out that should also be addressed in future studies regarding this topic would be the development of more studies exploring protective factors.
Despite the limitations, the multiple identified and discussed factors regarding relapse and rehospitalization show the importance of the tracking and assessment of these elements by clinical practitioners, aiming to not only plan interventions capable of promoting actual long-term results for a person with SUD but also cover individual, familiar, and social factors that surpass pharmacological treatment protocols. Considering the overview of most treatment modalities proposed in the reviewed studies, it begs the question of whether the dropout rates are due to a lack of more different types of treatment, lack of search by the users for better-suited treatment, or other clinical factors variables. Multidisciplinary-based interventions could aid in this process, including focusing on family, community, and social support. SUD health care treatments should be incentivized to address the evaluation of all categories reported here as part of routine clinical care. It also points out that individual characteristics such as early onset use (Viola et al., 2014), number of rehospitalizations (Proctor et al., 2022), or polysubstance use profile (Levola et al., 2021) may yield consideration as a new focus on treating SUD collectively. Future studies may investigate the relationship between the specific type of drug, polysubstance use, and the risk for relapse and rehospitalization.